Cost-utility Analysis of Evoke Closed-loop Spinal Cord Stimulation for Chronic Back and Leg Pain
- PMID: 37440335
- PMCID: PMC10498882
- DOI: 10.1097/AJP.0000000000001146
Cost-utility Analysis of Evoke Closed-loop Spinal Cord Stimulation for Chronic Back and Leg Pain
Abstract
Objectives: The effectiveness of Evoke closed-loop spinal cord stimulation (CL-SCS), a novel modality of neurostimulation, has been demonstrated in a randomized controlled trial (RCT). The objective of this cost-utility analysis was to develop a de novo economic model to estimate the cost-effectiveness of Evoke CL-SCS when compared with open-loop SCS (OL-SCS) for the management of chronic back and leg pain.
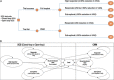
Methods: A decision tree followed by a Markov model was used to estimate the costs and outcomes of Evoke CL-SCS versus OL-SCS over a 15-year time horizon from the UK National Health Service perspective. A "high-responder" health state was included to reflect improved levels of SCS pain reduction recently reported. Results are expressed as incremental cost per quality-adjusted life year (QALY). Deterministic and probabilistic sensitivity analysis (PSA) was conducted to assess uncertainty in the model inputs.
Results: Evoke CL-SCS was estimated to be the dominant treatment strategy at ~5 years postimplant (ie, it generates more QALYs while cost saving compared with OL-SCS). Probabilistic sensitivity analysis showed that Evoke CL-SCS has a 92% likelihood of being cost-effective at a willingness to pay threshold of £20,000/QALY. Results were robust across a wide range of scenario and sensitivity analyses.
Discussion: The results indicate a strong economic case for the use of Evoke CL-SCS in the management of chronic back and leg pain with or without prior spinal surgery with dominance observed at ~5 years.
Copyright © 2023 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
This study was funded by Saluda Medical. R.V.D. has received consultancy fees from Mainstay Medical, Medtronic Ltd, and Saluda Medical. The work reported within this manuscript was finalized before R.V.D. joined Saluda Medical as an employee. AB MTech Access was commissioned by Saluda Medical to undertake this project. R.V.D., N.S., and A.L. are employees of Saluda Medical. A.G. has received honoraria for consulting as well as advisory board meetings for Nevro Corp, Boston Scientific Corp, and Abbott. P.S.S. serves as a consultant for Abbott, Nevro and Medtronic. He has received research support from Boston Scientific, Abbott, Vertos, Nevro, Saluda Medical and Grunenthal Halyard. D.S. is a consultant for Abbott, Boston Scientific, Flowonix, Medtronic, Nevro, PainTEQ, SPR Therapeutics, Vertos, and Vertiflex. S.M.F. serves as a consultant for Abbott, Medtronic, Nevro, Saluda, SPR, and Vertiflex. He has received research support from Abbott, Medtronic, Nuvectra, and Saluda Medical. C.W.H. has ownership in Axonics, PainTEQ, Nalu, Spine BioPharma, and Mainstay; consults for Saluda Medical and Genecentrix; is on the speaker’s bureau for Tersera; has research funding from Abbott, Biotronik, DiscGenics, Mesoblast, Saluda, TissueGene, and Vivex; and serves on the advisory boards of Abbott, Mainstay, Nalu, Spine BioPharma, and Vertiflex. R.S.T. has received consultancy fees from Medtronic Ltd, Nevro Corp, and Saluda Medical. The remaining author declares no conflict of interest.
Figures
References
-
- GBD. 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211–1259. - PMC - PubMed
-
- Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain. 2000;84:95–103. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous