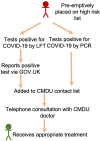
Evolving role of novel COVID-19 Medicine Delivery Units
- PMID: 37440865
- PMCID: PMC10241034
- DOI: 10.1136/ihj-2022-000135
Evolving role of novel COVID-19 Medicine Delivery Units
Keywords: COVID-19; evidence-based practice; health policy; health services research; prehospital care.
Conflict of interest statement
Competing interests: AG—principal investigator for the PANORAMIC, COV002, COV009, NOVAVAX, COMCOV2, COVBOOST and TICO trials at St Thomas’ Hospital, London. NS and AP—co-investigators for the PANORAMIC, COV002, COV009, NOVAVAX, COMCOV2, COVBOOST, ENSEMBLE-2, AZ-BOOST, RECOVERY, HEAL-COVID, SPRINTER, GILEAD and TICO trials at St Thomas’ Hospital, London.
Figures
References
-
- Interim clinical commissioning policy: antivirals or neutralising monoclonal antibodies for non-hospitalised patients with COVID-19 (version 5). England N; 2022. https://www.england.nhs.uk/coronavirus/wp-content/uploads/sites/52/2021/...
-
- MHpR A . Summary of Product Characteristics for Xevudy: Medicines & Healthcare products Regulatory Agency; 2021. Available: https://www.gov.uk/government/publications/regulatory-approval-of-xevudy...
LinkOut - more resources
Full Text Sources