Pregnancy-associated Steroid Effects on Insulin Sensitivity, Adipogenesis, and Lipogenesis: Role of Wnt/β-Catenin Pathway
- PMID: 37440965
- PMCID: PMC10334487
- DOI: 10.1210/jendso/bvad076
Pregnancy-associated Steroid Effects on Insulin Sensitivity, Adipogenesis, and Lipogenesis: Role of Wnt/β-Catenin Pathway
Abstract
Context: The shift in maternal energy metabolism characteristic of pregnancy is thought to be driven by various hormonal changes, especially of ovarian and placental steroids. Imbalances in circulating estradiol (E2) and progesterone (P4) levels during this period are often associated with metabolic disturbances leading to the development of gestational diabetes mellitus (GDM). Since abnormalities in the Wnt pathway effector transcription factor 7-like 2 (TCF7L2) are commonly associated with the occurrence of GDM, we hypothesized that the canonical or β-catenin-dependent Wnt signaling pathway mediates the metabolic actions of E2 and P4.
Objective: Our study was aimed at elucidating the metabolic function of the steroids E2 and P4, and examining the role of the canonical Wnt signaling pathway in mediating the actions of these steroids.
Methods: The ovariectomized (OVX) rat was used as a model system to study the effect of known concentrations of exogenously administered E2 and P4. Niclosamide (Nic) was administered to block Wnt signaling. 3T3-L1 cells were used to analyze changes in differentiation in the presence of the steroids or niclosamide.
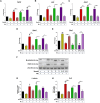
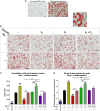
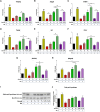
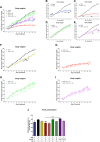
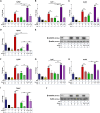
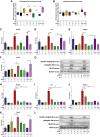
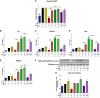
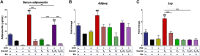
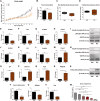
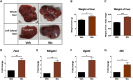
Results: In the present study, we observed that E2 enhanced insulin sensitivity and inhibited lipogenesis while P4 increased lipogenic gene expression-in 3T3-L1 adipocytes, and in adipose tissue and skeletal muscle of OVX rats when the dosage of E2 and P4 mimicked that of pregnancy. Both E2 and P4 were also found to upregulate Wnt signaling. Nic nhibited the steroid-mediated increase in Wnt signaling in adipocytes and OVX rats. The insulin-sensitizing and antilipogenic actions of E2 were found to be mediated by the canonical Wnt pathway, but the effects of P4 on lipogenesis appeared to be independent of it. Additionally, it was observed that inhibition of Wnt signaling by Nic hastened adipogenic differentiation, and the inhibitory effect of E2 on differentiation was prevented by Nic.
Conclusion: The findings presented in this study highlight the role of steroids and Wnt pathway in glucose and lipid metabolism and are relevant to understanding the pathophysiology of metabolic disorders arising from hormonal disturbances.
Keywords: adipose tissue; estradiol; insulin signaling; lipid metabolism; progesterone; skeletal muscle.
© The Author(s) 2023. Published by Oxford University Press on behalf of the Endocrine Society.
Figures


References
-
- Vejrazkova D, Vcelak J, Vankova M, et al. Steroids and insulin resistance in pregnancy. J Steroid Biochem Mol Biol. 2014;139:122‐129. - PubMed
-
- Del Rincon JP, Iida K, Gaylinn BD, et al. Growth hormone regulation of p85α expression and phosphoinositide 3-kinase activity in adipose tissue: mechanism for growth hormone-mediated insulin resistance. Diabetes. 2007;56(6):1638‐1646. - PubMed
-
- Masuyama H, Hiramatsu Y. Potential role of estradiol and progesterone in insulin resistance through constitutive androstane receptor. J Mol Endocrinol. 2011;47(2):229‐239. - PubMed
LinkOut - more resources
Full Text Sources
