Long-term safety and effectiveness of eculizumab in patients with aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder: a 2-year interim analysis of post-marketing surveillance in Japan
- PMID: 37441104
- PMCID: PMC10333632
- DOI: 10.1177/17562864231181177
Long-term safety and effectiveness of eculizumab in patients with aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder: a 2-year interim analysis of post-marketing surveillance in Japan
Abstract
Background: The terminal complement C5 inhibitor eculizumab is approved in Japan for relapse prevention in aquaporin-4 antibody-positive (AQP4+) neuromyelitis optica spectrum disorder (NMOSD) and is undergoing mandatory post-marketing surveillance (PMS) of clinical use.
Objectives: The objective of the study is to assess the real-world, long-term safety and effectiveness of eculizumab in Japanese patients with AQP4+ NMOSD.
Design: Regulatory-mandated PMS analysis implemented as an all-case surveillance of all patients with AQP4+ NMOSD who have been treated with eculizumab in Japan since its approval in November 2019.
Methods: This PMS interim analysis assessed the safety and effectiveness of eculizumab in Japanese patients with AQP4+ NMOSD from November 2019 to April 2022.
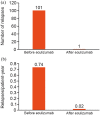
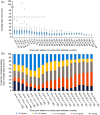
Results: Of 147 patients treated with eculizumab who consented to publication, 71 had at least one case report form collected and locked at the interim analysis data cut-off, constituting the safety analysis set; three patients from PREVENT (NCT01892345) were excluded from the effectiveness analysis set. Twelve and 10 patients in the safety and effectiveness analysis sets discontinued, respectively. In the safety analysis set, 67/71 patients (94.4%) were female, mean illness duration was 6.8 [standard deviation (SD): 6.2] years, mean age at eculizumab initiation was 50.7 (SD: 13.3) years, and mean eculizumab treatment duration was 44.6 (SD: 23.7) weeks. At diagnosis of NMOSD, 34/71 patients (47.9%) and 35/71 patients (49.3%) in the safety analysis set had symptoms of optic neuritis and transverse myelitis, respectively. In the safety analysis set, 19/71 patients (26.8%) reported adverse events, 10/71 (14.1%) reported adverse drug reactions (ADRs), and 7/71 (9.9%) reported serious ADRs; no meningococcal infections were observed. In the effectiveness analysis set, 64/68 patients (94.1%) were female, mean disease duration was 6.9 (SD: 6.3) years, mean age at eculizumab initiation was 50.6 (SD: 13.2) years, and 27/68 (39.7%) were tested for C5 genetic polymorphism (all negative). In the 2 years before eculizumab, 51/68 patients (75.0%) experienced relapse. Relapse rate was 0.02/patient-year after eculizumab initiation versus 0.74/patient-year in the 2 years before eculizumab. Overall, 37/68 patients (54.4%) were prescribed immunosuppressants in the 6 months before and 19/40 (47.5%) in the 6-12 months after starting eculizumab treatment. The proportion of patients taking >10 mg/day of prednisolone decreased from 45.6% at 24-20 weeks before to 23.1% and 0% at 48-52 and 100-104 weeks after eculizumab, respectively.
Conclusion: This article reports interim PMS data for Japanese patients and provides updated real-world evidence for the safety of eculizumab and its effectiveness at preventing relapses in patients with AQP4+ NMOSD. Safety and effectiveness results are consistent with those from PREVENT.
Keywords: aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder; eculizumab; post-marketing surveillance; real-world evidence; relapse prevention.
© The Author(s), 2023.
Conflict of interest statement
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ichiro Nakashima reports personal fees from Alexion Pharma GK, Chugai, Biogen Japan, Mitsubishi Tanabe Pharma, and Novartis, and grants from LSI Medience, the Ministry of Education, Science and Technology of Japan, and the Ministry of Health, Labor and Welfare of Japan (MHLW). Jin Nakahara reports personal fees from AbbVie, Alexion Pharma GK, Asahi Kasei Medical, Biogen, Bristol Myers Squibb, Chugai, CSL Behring, Daiichi Sankyo, Eisai, Kyorin, Mitsubishi Tanabe Pharma, Novartis, Otsuka, Roche, Takeda, and Teijin Pharma; research scholarships from AbbVie, Boehringer Ingelheim, Chugai, Daiichi Sankyo, EA Pharma, Eisai, JB, Mitsubishi Tanabe Pharma, Otsuka, Shionogi, Sumitomo Pharma, Teijin Pharma, and Tsumura; and grants from the Ministry of Education, Science and Technology of Japan, the MHLW, and Biogen. Hiroaki Yokote reports personal fees from Biogen Japan, Mitsubishi Tanabe Pharma, Novartis, Chugai Pharma, and Alexion Pharma GK; and grants from the MHLW. Yasuhiro Manabe declared no potential conflicts of interest with respect to this work. Kazumi Okamura and Kou Hasegawa are employees of, and hold stock in, Alexion Pharma GK, AstraZeneca Rare Disease. Kazuo Fujihara has received personal fees and other support from AbbVie, Asahi Kasei Medical, Biogen, Chugai, Eisai, Merck Group, Mitsubishi Tanabe Pharma, Novartis, Ono, Roche, Sumitomo Dainippon, Takeda, Teijin Pharma, UCB, and Viela Bio (formerly MedImmune); and grants from the Ministry of Education, Science and Technology of Japan and the MHLW.
Figures
References
-
- Wingerchuk DM, Hogancamp WF, O’Brien PC, et al.. The clinical course of neuromyelitis optica (Devic’s syndrome). Neurology 1999; 53: 1107–1114. - PubMed
-
- Wingerchuk DM, Lennon VA, Lucchinetti CF, et al.. The spectrum of neuromyelitis optica. Lancet Neurol 2007; 6: 805–815. - PubMed
-
- Fujihara K, Misu T, Nakashima I, et al.. Neuromyelitis optica should be classified as an astrocytopathic disease rather than a demyelinating disease. Clin Exp Neuroimmunol 2012; 3: 58–73.
LinkOut - more resources
Full Text Sources
Miscellaneous

