Accuracy of a faecal immunochemical test in patients under colonoscopy surveillance of colorectal adenoma and cancer
- PMID: 37441110
- PMCID: PMC10335070
- DOI: 10.48101/ujms.v128.8869
Accuracy of a faecal immunochemical test in patients under colonoscopy surveillance of colorectal adenoma and cancer
Abstract
Background: Surveillance of colorectal neoplasia place great strain on colonoscopy resources, and faecal immunochemical tests (FIT) are under-investigated for this purpose. The aim of this study was to report the outcome of FIT among patients scheduled for post-polypectomy and post-resection colorectal cancer (CRC) surveillance.
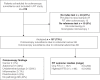
Methods: Patients scheduled for colonoscopy surveillance at five endoscopy units in mid-Sweden in 2016-2020 were eligible. They provided a faecal sample from 2 separate days, which were analysed by iFOBT QuikRead go® (Aidian Oy). Both the colonoscopies, and the FIT analyses were conducted by staff blinded to the other.
Results: Out of 216 included patients, 157 (73%) underwent both a complete colonoscopy and had at least one FIT analysed prior to the examination. The indication for surveillance was previous adenoma in 69 (44%) and post-resection CRC in 88 (56%) patients. Two (1%) in the CRC surveillance group were diagnosed with a metachronous CRC, whereas 49 (56%) patients in the CRC surveillance, and 17 (25%) in the adenoma group had no pathology identified at colonscopy (P < 0.001). The proportion of patients diagnosed with adenomas requiring surveillance according to European Society of Gastrointestinal Society (ESGE) guidelines 2020 was 6 (7%) in the post-CRC resection versus 7 (10%) in the adenoma surveillance group (P = 0.4). Based on one FIT and at cut-off 10 µg Hb/g, sensitivity for CRC was 100%, specificity 83% (95% confidence interval [CI]: 77-89), Positive Predictive Value (PPV) 7% (-2 to 16) and Negative Predictive Value (NPV) 100%. All patients with an adenoma requiring surveillance had a FIT below this cut-off. Adding a second FIT decreased the specificity.
Conclusion: Larger studies to evaluate the accuracy and consequences of using FIT for surveillance of colorectal neoplasia are needed. FIT may be more interesting for post-resection CRC surveillance than follow-up of adenoma.
Keywords: Colorectal cancer; adenoma; colonoscopy; faecal immunochemical test; surveillance.
© 2023 The Author(s). Published by Upsala Medical Society.
Conflict of interest statement
Aidian Oy provided a QuikRead go® instrument for analysis. The authors declare no conflict of interest.
References
-
- Maclean W, Zahoor Z, O’Driscoll S, Piggott C, Whyte MB, Rockall T, et al. Comparison of the QuikRead go((R)) point-of-care faecal immunochemical test for haemoglobin with the FOB Gold Wide((R)) laboratory analyser to diagnose colorectal cancer in symptomatic patients. Clin Chem Lab Med. 2022;60:101–8. doi: 10.1515/cclm-2021-0655 - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical