Coronary Artery Disease in Persons With Human Immunodeficiency Virus Without Detectable Viral Replication
- PMID: 37441354
- PMCID: PMC10334377
- DOI: 10.1093/ofid/ofad298
Coronary Artery Disease in Persons With Human Immunodeficiency Virus Without Detectable Viral Replication
Abstract
Background: We aimed to determine the prevalence of coronary artery disease (CAD) in persons with human immunodeficiency virus (HIV; PWH) and investigate whether inflammatory markers, including interleukin 6, IL-1β, and high-sensitivity C-reactive protein (hsCRP), were associated with CAD.
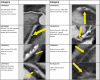
Methods: From the Copenhagen Comorbidity in HIV Infection (COCOMO) study, we included virologically suppressed PWH who underwent coronary computed tomographic (CT) angiography. Any atherosclerosis was defined as >0% stenosis, and obstructive CAD as ≥50% stenosis.
Results: Among 669 participants (mean age [standard deviation], 51 [11] years; 89% male), 300 (45%) had atherosclerosis, and 119 (18%) had obstructive CAD. The following risk factors were associated with any atherosclerosis and with obstructive CAD: age, male sex, hypertension, diabetes, smoking, dyslipidemia, time with HIV, and current protease inhibitor use. Interleukin 6 (IL-6) and hsCRP levels >2 mg/L were associated with any atherosclerosis and with obstructive CAD in univariable analyses but not after adjustment for traditional risk factors. IL-1β was not associated with CAD.
Conclusions: In a large population of PWH without viral replication, almost half had angiographically verified atherosclerosis. High concentrations of IL-6 and hsCRP were associated with CAD in univariable analyses, but adjustment for cardiovascular risk factors attenuated the association, suggesting that inflammation may mediate the association between traditional risk factors and CAD.
Keywords: CCTA; HIV; comorbidity; coronary artery disease; inflammation.
© The Author(s) 2023. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. A. D. K has received grants from the Danish Heart Foundation and the European Commission (the European Union’s Seventh Framework Programme; grant 603266). T. B reports grants from the Novo Nordisk Foundation, the Lundbeck Foundation, the Simonsen Foundation, GSK, Pfizer, Gilead, the Kai Hansen Foundation, and Erik and Susanna Olesen's Charitable Fund and personal fees from GSK, Pfizer, Boehringer Ingelheim, Gilead, MSD, Pentabase, Becton Dickinson, Janssen, and Astra Zeneca, all outside the submitted work. L. K. has received speaker honoraria from Novo, Novartis, AstraZeneca, Boehringer, and Bayer, unrelated to the current work. K. F. K. has received research grants from AP Møller og Hustru Chastine McKinney Møllers Fond, the Research Council of Rigshopitalet, the University of Copenhagen, the Danish Heart Foundation, the Danish Agency for Science, Technology and Innovation of the Danish Council for Strategic Research, the Novo Nordisk Foundation, Canon Medical Systems, and GE Healthcare. S. D. N. has received unrestricted research grants from the Novo Nordisk Foundation, the Lundbeck Foundation, the Augustinus Foundation, and the Rigshospitalet Research Council and reports advisory board activity for Gilead and GSK/ViiV, all unrelated to the current work. All other authors report no potential conflicts.
Figures


References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

