Cardiac magnetic resonance of hypertrophic heart phenotype: A review
- PMID: 37441401
- PMCID: PMC10333467
- DOI: 10.1016/j.heliyon.2023.e17336
Cardiac magnetic resonance of hypertrophic heart phenotype: A review
Abstract
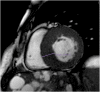
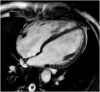
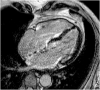
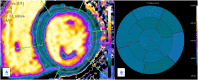
Hypertrophic heart phenotype is characterized by an abnormal left ventricular (LV) thickening. A hypertrophic phenotype can develop as adaptive response in many different conditions such as aortic stenosis, hypertension, athletic training, infiltrative heart muscle diseases, storage disorders and metabolic disorders. Hypertrophic cardiomyopathy (HCM) is the most frequent primary cardiomyopathy (CMP) and a genetical cause of cardiac hypertrophy. It requires the exclusion of any other cause of LV hypertrophy. Cardiac magnetic resonance (CMR) is a comprehensive imaging technique that allows a detailed evaluation of myocardial diseases. It provides reproducible measurements and myocardial tissue characterization. In clinical practice CMR is increasingly used to confirm the presence of ventricular hypertrophy, to detect the underlying cause of the phenotype and more recently as an efficient prognostic tool. This article aims to provide a detailed overview of the applications of CMR in the setting of hypertrophic heart phenotype and its role in the diagnostic workflow of such condition.
Keywords: Amyloidosis; Anderson-fabry disease; CMR; Cardiac hypertrophy; HCM; Iron overload; Sarcoidosis.
© 2023 Published by Elsevier Ltd.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Marco Gatti MD is a Guest editor for Heliyon Clinical Research.
Figures







References
-
- Baggiano A., del Torto A., Guglielmo M., Muscogiuri G., Fusini L., Babbaro M., Collevecchio A., Mollace R., Scafuri S., Mushtaq S., Conte E., Annoni A.D., Formenti A., Mancini M.E., Mostardini G., Andreini D., Guaricci A.I., Pepi M., Fontana M., Pontone G. Role of CMR mapping techniques in cardiac hypertrophic phenotype. Diagnostics. 2020;10 doi: 10.3390/diagnostics10100770. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

