The Effects of Peroxisome Proliferator-Activated Receptor-Delta Modulator ASP1128 in Patients at Risk for Acute Kidney Injury Following Cardiac Surgery
- PMID: 37441472
- PMCID: PMC10334402
- DOI: 10.1016/j.ekir.2023.04.004
The Effects of Peroxisome Proliferator-Activated Receptor-Delta Modulator ASP1128 in Patients at Risk for Acute Kidney Injury Following Cardiac Surgery
Abstract
Introduction: Peroxisome proliferator-activated receptor δ (PPARδ) plays a central role in modulating mitochondrial function in ischemia-reperfusion injury. The novel PPARδ modulator, ASP1128, was evaluated.
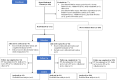
Methods: A randomized, double-blind, placebo-controlled, biomarker assignment-driven, multicenter study was performed in adult patients at risk for acute kidney injury (AKI) following cardiac surgery, examining efficacy and safety of a 3-day, once-daily intravenous dose of 100 mg ASP1128 versus placebo (1:1). AKI risk was based on clinical characteristics and postoperative urinary biomarker (TIMP2)•(IGFBP7). The primary end point was the proportion of patients with AKI based on serum creatinine within 72 hours postsurgery (AKI-SCr72h). Secondary endpoints included the composite end point of major adverse kidney events (MAKE: death, renal replacement therapy, and/or ≥25% reduction of estimated glomerular filtration rate [eGFR]) at days 30 and 90).
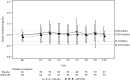
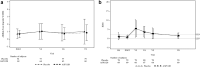
Results: A total of 150 patients were randomized and received study medication (81 placebo, 69 ASP1128). Rates of AKI-SCr72h were 21.0% and 24.6% in the placebo and ASP1128 arms, respectively (P = 0.595). Rates of moderate/severe AKI (stage 2/3 AKI-SCr and/or stage 3 AKI-urinary output criteria) within 72 hours postsurgery were 19.8% and 23.2%, respectively (P = 0.609). MAKE occurred within 30 days in 11.1% and 13.0% in the placebo and ASP1128 arms (P = 0.717), respectively; and within 90 days in 9.9% and 15.9% in the placebo and ASP1128 arms (P = 0.266), respectively. No safety issues were identified with ASP1128 treatment, but rates of postoperative atrial fibrillation were lower (11.6%) than in the placebo group (29.6%).
Conclusion: ASP1128 was safe and well-tolerated in patients at risk for AKI following cardiac surgery, but it did not show efficacy in renal endpoints.
Keywords: PPAR delta; acute kidney injury; cardiac surgical procedures; controlled trial; randomized; reperfusion injury.
© 2023 International Society of Nephrology. Published by Elsevier Inc.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

