Neurogenic Defects Occur in LRIG2-Associated Urinary Bladder Disease
- PMID: 37441484
- PMCID: PMC10334403
- DOI: 10.1016/j.ekir.2023.04.017
Neurogenic Defects Occur in LRIG2-Associated Urinary Bladder Disease
Abstract
Introduction: Urofacial, or Ochoa, syndrome (UFS) is an autosomal recessive disease featuring a dyssynergic bladder with detrusor smooth muscle contracting against an undilated outflow tract. It also features an abnormal grimace. Half of individuals with UFS carry biallelic variants in HPSE2, whereas other rare families carry variants in LRIG2.LRIG2 is immunodetected in pelvic ganglia sending autonomic axons into the bladder. Moreover, Lrig2 mutant mice have abnormal urination and abnormally patterned bladder nerves. We hypothesized that peripheral neurogenic defects underlie LRIG2-associated bladder dysfunction.
Methods: We describe a new family with LRIG2-associated UFS and studied Lrig2 homozygous mutant mice with ex vivo physiological analyses.
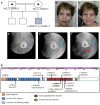
Results: The index case presented antenatally with urinary tract (UT) dilatation, and postnatally had urosepsis and functional bladder outlet obstruction. He had the grimace that, together with UT disease, characterizes UFS. Although HPSE2 sequencing was normal, he carried a homozygous, predicted pathogenic, LRIG2 stop variant (c.1939C>T; p.Arg647∗). Lrig2 mutant mice had enlarged bladders. Ex vivo physiology experiments showed neurogenic smooth muscle relaxation defects in the outflow tract, containing the urethra adjoining the bladder, and in detrusor contractility. Moreover, there were nuanced differences in physiological outflow tract defects between the sexes.
Conclusion: Putting this family in the context of all reported UT disease-associated LRIG2 variants, the full UFS phenotype occurs with biallelic stop or frameshift variants, but missense variants lead to bladder-limited disease. Our murine observations support the hypothesis that UFS is a genetic autonomic neuropathy of the bladder affecting outflow tract and bladder body function.
Keywords: LRIG2; Ochoa; bladder; neurogenic; syndrome; urofacial.
© 2023 International Society of Nephrology. Published by Elsevier Inc.
Figures




References
Grants and funding
LinkOut - more resources
Full Text Sources

