Current and potential roles of immuno-PET/-SPECT in CAR T-cell therapy
- PMID: 37441689
- PMCID: PMC10333708
- DOI: 10.3389/fmed.2023.1199146
Current and potential roles of immuno-PET/-SPECT in CAR T-cell therapy
Abstract
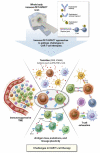
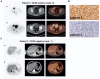
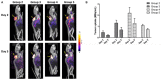
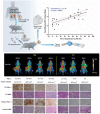
Chimeric antigen receptor (CAR) T-cell therapies have evolved as breakthrough treatment options for the management of hematological malignancies and are also being developed as therapeutics for solid tumors. However, despite the impressive patient responses from CD19-directed CAR T-cell therapies, ~ 40%-60% of these patients' cancers eventually relapse, with variable prognosis. Such relapses may occur due to a combination of molecular resistance mechanisms, including antigen loss or mutations, T-cell exhaustion, and progression of the immunosuppressive tumor microenvironment. This class of therapeutics is also associated with certain unique toxicities, such as cytokine release syndrome, immune effector cell-associated neurotoxicity syndrome, and other "on-target, off-tumor" toxicities, as well as anaphylactic effects. Furthermore, manufacturing limitations and challenges associated with solid tumor infiltration have delayed extensive applications. The molecular imaging modalities of immunological positron emission tomography and single-photon emission computed tomography (immuno-PET/-SPECT) offer a target-specific and highly sensitive, quantitative, non-invasive platform for longitudinal detection of dynamic variations in target antigen expression in the body. Leveraging these imaging strategies as guidance tools for use with CAR T-cell therapies may enable the timely identification of resistance mechanisms and/or toxic events when they occur, permitting effective therapeutic interventions. In addition, the utilization of these approaches in tracking the CAR T-cell pharmacokinetics during product development and optimization may help to assess their efficacy and accordingly to predict treatment outcomes. In this review, we focus on current challenges and potential opportunities in the application of immuno-PET/-SPECT imaging strategies to address the challenges encountered with CAR T-cell therapies.
Keywords: CAR T-cell therapy; cell therapy; immuno-PET; immuno-SPECT; tumor microenvironment.
Copyright © 2023 Mulgaonkar, Udayakumar, Yang, Harris, Öz, Ramakrishnan Geethakumari and Sun.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
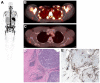
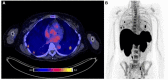
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

