Tissue characterization of benign cardiac tumors by cardiac magnetic resonance imaging, a review of core imaging protocol and benign cardiac tumors
- PMID: 37441708
- PMCID: PMC10333494
- DOI: 10.3389/fcvm.2023.1009411
Tissue characterization of benign cardiac tumors by cardiac magnetic resonance imaging, a review of core imaging protocol and benign cardiac tumors
Abstract
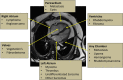
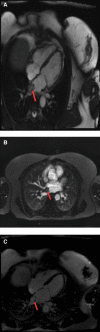
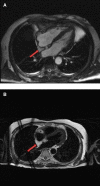
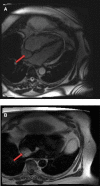
Generally, cardiac masses are initially suspected on routine echocardiography. Cardiac magnetic resonance (CMR) imaging is further performed to differentiate tumors from pseudo-tumors and to characterize the cardiac masses based on their appearance on T1/T2-weighted images, detection of perfusion and demonstration of gadolinium-based contrast agent uptake on early and late gadolinium enhancement images. Further evaluation of cardiac masses by CMR is critical because unnecessary surgery can be avoided by better tissue characterization. Different cardiac tissues have different T1 and T2 relaxation times, principally owing to different internal biochemical environments surrounding the protons. In CMR, the signal intensity from a particular tissue depends on its T1 and T2 relaxation times and its proton density. CMR uses this principle to differentiate between various tissue types by weighting images based on their T1 or T2 relaxation times. Generally, tumor cells are larger, edematous, and have associated inflammatory reactions. Higher free water content of the neoplastic cells and other changes in tissue composition lead to prolonged T1/T2 relaxation times and thus an inherent contrast between tumors and normal tissue exists. Overall, these biochemical changes create an environment where different cardiac masses produce different signal intensity on their T1- weighted and T2- weighted images that help to discriminate between them. In this review article, we have provided a detailed description of the core CMR imaging protocol for evaluation of cardiac masses. We have also discussed the basic features of benign cardiac tumors as well as the role of CMR in evaluation and further tissue characterization of these tumors.
Keywords: atrial myxoma; benign cardiac tumors; cardiac MRI (CMR); fibroelastoma; magnetic resonance imaging; tissue characterization.
© 2023 Haider, Ullah, Fatima, Karim, Haq, Majid, Anwar, Nawaz, Ehsan, Ali, Sarwar, Anwar, Khan, Humayun and Alam.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


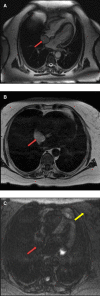
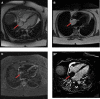

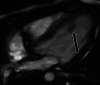
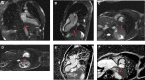
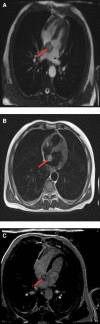
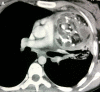
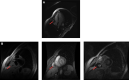
References
-
- Sütsch G, Jenni R, von Segesser L, Schneider J. Heart tumors: incidence, distribution, diagnosis exemplified by 20,305 echocardiographies. Schweiz Med Wochenschr. (1991) 121:621–29. PMID: 2047823 - PubMed
Publication types
LinkOut - more resources
Full Text Sources

