Net-1,2-Hydrogen Atom Transfer of Amidyl Radicals: Toward the Synthesis of 1,2-Diamine Derivatives
- PMID: 37441806
- PMCID: PMC10411589
- DOI: 10.1021/jacs.3c04376
Net-1,2-Hydrogen Atom Transfer of Amidyl Radicals: Toward the Synthesis of 1,2-Diamine Derivatives
Abstract
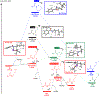
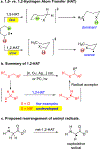
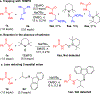
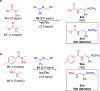
Hydrogen atom transfer (HAT) processes are among the most useful approaches for the selective construction of C(sp3)-C(sp3) bonds. 1,5-HAT with heteroatom-centered radicals (O•, N•) have been well established and are favored relative to other 1,n-HAT processes. In comparison, net 1,2-HAT processes have been observed infrequently. Herein, the first amidyl radicalls are reported that preferentially undergo a net 1,2-HAT over 1,5-HAT. Beginning with single electron transfer from 2-azaallyl anions to N-alkyl N-aryloxy amides, the latter generate amidyl radicals. The amidyl radical undergoes a net-1,2-HAT to generate a C-centered radical that participates in an intermolecular radical-radical coupling with the 2-azaallyl radical to generate 1,2-diamine derivatives. Mechanistic and EPR experiments point to radical intermediates. Density functional theory calculations provide support for a base-assisted, stepwise-1,2-HAT process. It is proposed that the generation of amidyl radicals under basic conditions can be greatly expanded to access α-amino C-centered radicals that will serve as valuable synthetic intermediates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





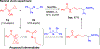


Similar articles
-
Visible-light-driven net-1,2-hydrogen atom transfer of amidyl radicals to access β-amido ketone derivatives.Chem Sci. 2024 Dec 3;16(2):962-969. doi: 10.1039/d4sc04997g. eCollection 2025 Jan 2. Chem Sci. 2024. PMID: 39664809 Free PMC article.
-
Benzylic C(sp3)-H/C(sp3)-H Coupling with 2-Azaallyl Anions through Single-Electron Transfer and 1,5-Hydrogen Atom Transfer.Org Lett. 2025 Mar 28;27(12):3054-3059. doi: 10.1021/acs.orglett.5c00708. Epub 2025 Mar 19. Org Lett. 2025. PMID: 40108942
-
Catalytic alkylation of remote C-H bonds enabled by proton-coupled electron transfer.Nature. 2016 Nov 10;539(7628):268-271. doi: 10.1038/nature19811. Epub 2016 Oct 12. Nature. 2016. PMID: 27732585 Free PMC article.
-
Recent advances in amidyl radical-mediated photocatalytic direct intermolecular hydrogen atom transfer.Beilstein J Org Chem. 2025 Jun 27;21:1306-1323. doi: 10.3762/bjoc.21.100. eCollection 2025. Beilstein J Org Chem. 2025. PMID: 40589434 Free PMC article. Review.
-
C-H functionalization reactions enabled by hydrogen atom transfer to carbon-centered radicals.Chem Sci. 2020 Nov 16;11(48):12974-12993. doi: 10.1039/d0sc04881j. Chem Sci. 2020. PMID: 34123240 Free PMC article. Review.
Cited by
-
Efficient construction of functionalized pyrroloindolines through cascade radical cyclization/intermolecular coupling.Chem Sci. 2024 Jan 4;15(6):2205-2210. doi: 10.1039/d3sc05210a. eCollection 2024 Feb 7. Chem Sci. 2024. PMID: 38332810 Free PMC article.
-
Visible-light-driven net-1,2-hydrogen atom transfer of amidyl radicals to access β-amido ketone derivatives.Chem Sci. 2024 Dec 3;16(2):962-969. doi: 10.1039/d4sc04997g. eCollection 2025 Jan 2. Chem Sci. 2024. PMID: 39664809 Free PMC article.
References
-
- White MC, Adding Aliphatic C−H Bond Oxidations to Synthesis. Science 2012, 335 (6070), 807–809. - PubMed
-
- Huang Z; Lim HN; Mo F; Young MC; Dong G, Transition metal-catalyzed ketone-directed or mediated C-H functionalization. Chem. Soc. Rev 2015, 44 (21), 7764–7786. - PubMed
-
- Yamaguchi J; Yamaguchi AD; Itami K, Funktionalisierung von C-H-Bindungen: neue Synthesemethoden für Naturstoffe und Pharmazeutika. Angew. Chem 2012, 124 (36), 9092–9142.
Grants and funding
LinkOut - more resources
Full Text Sources

