Net-1,2-Hydrogen Atom Transfer of Amidyl Radicals: Toward the Synthesis of 1,2-Diamine Derivatives
- PMID: 37441806
- PMCID: PMC10411589
- DOI: 10.1021/jacs.3c04376
Net-1,2-Hydrogen Atom Transfer of Amidyl Radicals: Toward the Synthesis of 1,2-Diamine Derivatives
Abstract
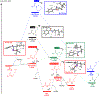
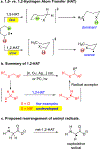
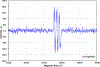
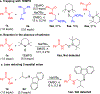
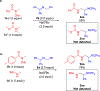
Hydrogen atom transfer (HAT) processes are among the most useful approaches for the selective construction of C(sp3)-C(sp3) bonds. 1,5-HAT with heteroatom-centered radicals (O•, N•) have been well established and are favored relative to other 1,n-HAT processes. In comparison, net 1,2-HAT processes have been observed infrequently. Herein, the first amidyl radicalls are reported that preferentially undergo a net 1,2-HAT over 1,5-HAT. Beginning with single electron transfer from 2-azaallyl anions to N-alkyl N-aryloxy amides, the latter generate amidyl radicals. The amidyl radical undergoes a net-1,2-HAT to generate a C-centered radical that participates in an intermolecular radical-radical coupling with the 2-azaallyl radical to generate 1,2-diamine derivatives. Mechanistic and EPR experiments point to radical intermediates. Density functional theory calculations provide support for a base-assisted, stepwise-1,2-HAT process. It is proposed that the generation of amidyl radicals under basic conditions can be greatly expanded to access α-amino C-centered radicals that will serve as valuable synthetic intermediates.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





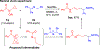


References
-
- White MC, Adding Aliphatic C−H Bond Oxidations to Synthesis. Science 2012, 335 (6070), 807–809. - PubMed
-
- Huang Z; Lim HN; Mo F; Young MC; Dong G, Transition metal-catalyzed ketone-directed or mediated C-H functionalization. Chem. Soc. Rev 2015, 44 (21), 7764–7786. - PubMed
-
- Yamaguchi J; Yamaguchi AD; Itami K, Funktionalisierung von C-H-Bindungen: neue Synthesemethoden für Naturstoffe und Pharmazeutika. Angew. Chem 2012, 124 (36), 9092–9142.
Grants and funding
LinkOut - more resources
Full Text Sources

