Neoadjuvant treatment does not influence PD-L1 expression in stage III non-small-cell lung cancer: a retrospective analysis of tumor samples from the trials SAKK 16/96, 16/00, 16/01, and 16/14
- PMID: 37441877
- PMCID: PMC10515281
- DOI: 10.1016/j.esmoop.2023.101595
Neoadjuvant treatment does not influence PD-L1 expression in stage III non-small-cell lung cancer: a retrospective analysis of tumor samples from the trials SAKK 16/96, 16/00, 16/01, and 16/14
Abstract
Background: The inclusion of immune checkpoint inhibitors (ICIs) in the treatment of operable stage III non-small-cell lung cancer is becoming a new standard. Programmed death-ligand 1 (PD-L1) protein expression on tumor cells has emerged as the most important biomarker for sensitivity to ICIs targeting the programmed cell death protein 1 (PD-1)-PD-L1 axis. Little is known about the impact of neoadjuvant treatment on PD-L1 expression.
Patients and methods: We assessed PD-L1 expression by immunohistochemistry (Ventana SP263 assay) on tumor cells in treatment-naive diagnostic tumor samples and matched lung resections from patients with stage III non-small-cell lung cancer included in the Swiss Group for Clinical Cancer Research (SAKK) trials 16/96, 16/00, 16/01, and 16/14. All patients received neoadjuvant chemotherapy (CT) with cisplatin/docetaxel, either as single modality (CT), with sequential radiotherapy [chemoradiation therapy (CRT)] or with the PD-L1 inhibitor durvalumab (CT + ICI).
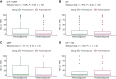
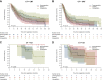
Results: Overall, 132 paired tumor samples were analyzed from patients with neoadjuvant CT (n = 69), CRT (n = 33) and CT + ICI (n = 30). For CT and CRT, PD-L1 expression before and after neoadjuvant treatment did not differ significantly (Wilcoxon test, P = 0.94). Likewise, no statistically significant difference was observed between CT and CRT for PD-L1 expression after neoadjuvant treatment (P = 0.97). For CT + ICI, PD-L1 expression before and after neoadjuvant treatment also did not differ significantly (Wilcoxon test, P > 0.99). Event-free survival and overall survival for patients with downregulation or upregulation of PD-L1 expression after neoadjuvant treatment were similar.
Conclusions: In our cohort of patients neoadjuvant treatment did not influence PD-L1 expression, irrespective of the specific neoadjuvant treatment protocol. Dynamic change of PD-L1 expression did not correlate with event-free survival or overall survival.
Keywords: PD-L1 expression; chemoradiation; immune checkpoint inhibitor; non-small-cell lung cancer; predictive biomarker.
Copyright © 2023 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures


References
-
- Reck M., Rodriguez-Abreu D., Robinson A.G., et al. Pembrolizumab versus chemotherapy for PD-L1-positive non-small-cell lung cancer. N Engl J Med. 2016;375(19):1823–1833. - PubMed
-
- Gandhi L., Rodriguez-Abreu D., Gadgeel S., et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N Engl J Med. 2018;378(22):2078–2092. - PubMed
-
- Paz-Ares L., Luft A., Vicente D., et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N Engl J Med. 2018;379(21):2040–2051. - PubMed
-
- Felip E., Altorki N., Zhou C., et al. Adjuvant atezolizumab after adjuvant chemotherapy in resected stage IB-IIIA non-small-cell lung cancer (IMpower010): a randomised, multicentre, open-label, phase 3 trial. Lancet. 2021;398(10308):1344–1357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

