Sorcin promotes migration in cancer and regulates the EGF-dependent EGFR signaling pathways
- PMID: 37442828
- PMCID: PMC10345051
- DOI: 10.1007/s00018-023-04850-4
Sorcin promotes migration in cancer and regulates the EGF-dependent EGFR signaling pathways
Abstract
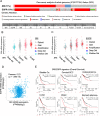
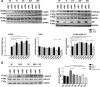
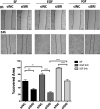
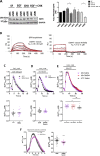
The epidermal growth factor receptor (EGFR) is one of the main tumor drivers and is an important therapeutic target for many cancers. Calcium is important in EGFR signaling pathways. Sorcin is one of the most important calcium sensor proteins, overexpressed in many tumors, that promotes cell proliferation, migration, invasion, epithelial-to-mesenchymal transition, malignant progression and resistance to chemotherapeutic drugs. The present work elucidates a functional mechanism that links calcium homeostasis to EGFR signaling in cancer. Sorcin and EGFR expression are significantly correlated and associated with reduced overall survival in cancer patients. Mechanistically, Sorcin directly binds EGFR protein in a calcium-dependent fashion and regulates calcium (dys)homeostasis linked to EGF-dependent EGFR signaling. Moreover, Sorcin controls EGFR proteostasis and signaling and increases its phosphorylation, leading to increased EGF-dependent migration and invasion. Of note, silencing of Sorcin cooperates with EGFR inhibitors in the regulation of migration, highlighting calcium signaling pathway as an exploitable target to enhance the effectiveness of EGFR-targeting therapies.
Keywords: Calcium; Cancer; EGF; EGFR; Epithelial-to-mesenchymal transition; Invasion; Migration; Signaling; Sorcin.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

