Fibronectin-binding molecules of Scedosporium apiospermum: focus on adhesive events
- PMID: 37442880
- PMCID: PMC10689634
- DOI: 10.1007/s42770-023-01062-7
Fibronectin-binding molecules of Scedosporium apiospermum: focus on adhesive events
Abstract
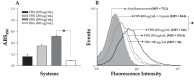
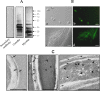
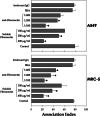
Scedosporium apiospermum is a widespread, emerging, and multidrug-resistant filamentous fungus that can cause localized and disseminated infections. The initial step in the infection process involves the adhesion of the fungus to host cells and/or extracellular matrix components. However, the mechanisms of adhesion involving surface molecules in S. apiospermum are not well understood. Previous studies have suggested that the binding of fungal receptors to fibronectin enhances its ability to attach to and infect host cells. The present study investigated the effects of fibronectin on adhesion events of S. apiospermum. The results revealed that conidial cells were able to bind to both immobilized and soluble human fibronectin in a typically dose-dependent manner. Moreover, fibronectin binding was virtually abolished in trypsin-treated conidia, suggesting the proteinaceous nature of the binding site. Western blotting assay, using fibronectin and anti-fibronectin antibody, evidenced 7 polypeptides with molecular masses ranging from 55 to 17 kDa in both conidial and mycelial extracts. Fibronectin-binding molecules were localized by immunofluorescence and immunocytochemistry microscopies at the cell wall and in intracellular compartments of S. apiospermum cells. Furthermore, a possible function for the fibronectin-like molecules of S. apiospermum in the interaction with host lung cells was assessed. Conidia pre-treated with soluble fibronectin showed a significant reduction in adhesion to either epithelial or fibroblast lung cells in a classically dose-dependent manner. Similarly, the pre-treatment of the lung cells with anti-fibronectin antibodies considerably diminished the adhesion. Collectively, the results demonstrated the presence of fibronectin-binding molecules in S. apiospermum cells and their role in adhesive events.
Keywords: Adhesion; Fibronectin; Infection; Lung cells; Scedosporium; Virulence.
© 2023. The Author(s) under exclusive licence to Sociedade Brasileira de Microbiologia.
Conflict of interest statement
The authors declare no competing interests.
Figures
References
-
- Cortez KJ, Roilides E, Quiroz-Telles F, Meletiadis J, Antachopoulos C, Knudsen T, Buchanan W, Milanovich J, Sutton DA, Fothergill A, Rinaldi MG, Shea YR, Zaoutis T, Kottilil S, Walsh TJ. Infections caused by Scedosporium spp. Clin Microbiol Rev. 2017;21(1):157–197. doi: 10.1128/CMR.00039-07. - DOI - PMC - PubMed
-
- Ramirez-Garcia A, Pellon A, Rementeria A, Buldain I, Barreto-Bergter E, Rollin-Pinheiro R, de Meirelles JV, Xisto MIDS, Ranque S, Havlicek V, Vandeputte P, Govic YL, Bouchara JP, Giraud S, Chen S, Rainer J, Alastruey-Izquierdo A, Martin-Gomez MT, López-Soria LM, Peman J, Schwarz C, Bernhardt A, Tintelnot K, Capilla J, Martin-Vicente A, Cano-Lira J, Nagl M, Lackner M, Irinyi L, Meyer W, de Hoog S, Hernando FL. Scedosporium and Lomentospora: an updated overview of underrated opportunists. Med Mycol. 2018;56(suppl_1):102–125. doi: 10.1093/mmy/myx113. - DOI - PubMed
-
- Mello TP, Bittencourt VCB, Liporagi-Lopes LC, Aor AC, Branquinha MH, Santos ALS. Insights into the social life and obscure side of Scedosporium/Lomentospora species: ubiquitous, emerging and multidrug-resistant opportunistic pathogens. Fungal Biol Rev. 2018;33:16–46. doi: 10.1016/j.fbr.2018.07.002. - DOI
-
- Aor AC, Mello TP, Sangenito LS, Fonseca BB, Rozental S, Lione VF, Veiga VF, Branquinha MH, Santos ALS. Ultrastructural viewpoints on the interaction events of Scedosporium apiospermum conidia with lung and macrophage cells. Mem Inst Oswaldo Cruz. 2018;113(10):e180311. doi: 10.1590/0074-02760180311. - DOI - PMC - PubMed
-
- Mello TP, Aor AC, Branquinha MH, Santos ALS. Insights into the interaction ofScedosporium apiospermum,Scedosporium aurantiacum,Scedosporium minutisporum, andLomentospora prolificanswith lung epithelial cells. Braz J Microbiol. 2019;51(2):427–436. doi: 10.1007/s42770-019-00183-2. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

