Using the LeiCNS-PK3.0 Physiologically-Based Pharmacokinetic Model to Predict Brain Extracellular Fluid Pharmacokinetics in Mice
- PMID: 37442882
- PMCID: PMC10733198
- DOI: 10.1007/s11095-023-03554-5
Using the LeiCNS-PK3.0 Physiologically-Based Pharmacokinetic Model to Predict Brain Extracellular Fluid Pharmacokinetics in Mice
Abstract
Introduction: The unbound brain extracelullar fluid (brainECF) to plasma steady state partition coefficient, Kp,uu,BBB, values provide steady-state information on the extent of blood-brain barrier (BBB) transport equilibration, but not on pharmacokinetic (PK) profiles seen by the brain targets. Mouse models are frequently used to study brain PK, but this information cannot directly be used to inform on human brain PK, given the different CNS physiology of mouse and human. Physiologically based PK (PBPK) models are useful to translate PK information across species.
Aim: Use the LeiCNS-PK3.0 PBPK model, to predict brain extracellular fluid PK in mice.
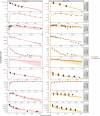
Methods: Information on mouse brain physiology was collected from literature. All available connected data on unbound plasma, brainECF PK of 10 drugs (cyclophosphamide, quinidine, erlotonib, phenobarbital, colchicine, ribociclib, topotecan, cefradroxil, prexasertib, and methotrexate) from different mouse strains were used. Dosing regimen dependent plasma PK was modelled, and Kpuu,BBB values were estimated, and provided as input into the LeiCNS-PK3.0 model to result in prediction of PK profiles in brainECF.
Results: Overall, the model gave an adequate prediction of the brainECF PK profile for 7 out of the 10 drugs. For 7 drugs, the predicted versus observed brainECF data was within two-fold error limit and the other 2 drugs were within five-fold error limit.
Conclusion: The current version of the mouse LeiCNS-PK3.0 model seems to reasonably predict available information on brainECF from healthy mice for most drugs. This brings the translation between mouse and human brain PK one step further.
Keywords: brain; leiCNS-PK3.0; mouse; physiologically-based pharmacokinetics (PBPK).
© 2023. The Author(s).
Conflict of interest statement
Not applicable.
Figures

Similar articles
-
Reliability of in vitro data for the mechanistic prediction of brain extracellular fluid pharmacokinetics of P-glycoprotein substrates in vivo; are we scaling correctly?J Pharmacokinet Pharmacodyn. 2025 Feb 8;52(2):16. doi: 10.1007/s10928-025-09963-w. J Pharmacokinet Pharmacodyn. 2025. PMID: 39921770 Free PMC article.
-
Exploring Kp,uu,BBB values smaller than unity in remoxipride: A physiologically-based CNS model approach highlighting brain metabolism in drugs with passive blood-brain barrier transport.Eur J Pharm Sci. 2024 Dec 1;203:106883. doi: 10.1016/j.ejps.2024.106883. Epub 2024 Aug 22. Eur J Pharm Sci. 2024. PMID: 39181172
-
Lumbar cerebrospinal fluid-to-brain extracellular fluid surrogacy is context-specific: insights from LeiCNS-PK3.0 simulations.J Pharmacokinet Pharmacodyn. 2021 Oct;48(5):725-741. doi: 10.1007/s10928-021-09768-7. Epub 2021 Jun 17. J Pharmacokinet Pharmacodyn. 2021. PMID: 34142308 Free PMC article.
-
Microdialysis: the Key to Physiologically Based Model Prediction of Human CNS Target Site Concentrations.AAPS J. 2017 Jul;19(4):891-909. doi: 10.1208/s12248-017-0050-3. Epub 2017 Mar 9. AAPS J. 2017. PMID: 28281195 Review.
-
Toward the prediction of CNS drug-effect profiles in physiological and pathological conditions using microdialysis and mechanism-based pharmacokinetic-pharmacodynamic modeling.AAPS J. 2005 Oct 7;7(3):E532-43. doi: 10.1208/aapsj070354. AAPS J. 2005. PMID: 16353931 Free PMC article. Review.
Cited by
-
Mathematical modeling of transdermal delivery of topical drug formulations in a dynamic microfluidic diffusion chamber in health and disease.PLoS One. 2024 Apr 11;19(4):e0299501. doi: 10.1371/journal.pone.0299501. eCollection 2024. PLoS One. 2024. PMID: 38603673 Free PMC article.
-
Reliability of in vitro data for the mechanistic prediction of brain extracellular fluid pharmacokinetics of P-glycoprotein substrates in vivo; are we scaling correctly?J Pharmacokinet Pharmacodyn. 2025 Feb 8;52(2):16. doi: 10.1007/s10928-025-09963-w. J Pharmacokinet Pharmacodyn. 2025. PMID: 39921770 Free PMC article.
References
-
- Murillo-Cuesta S, Artuch R, Asensio F, de la Villa P, Dierssen M, Enríquez JA, Fillat C, Fourcade S, Ibáñez B, Montoliu L, Oliver E, Pujol A, Salido E, Vallejo M, Varela-Nieto I. The value of mouse models of rare diseases: A spanish experience. Front Genet. 2020; 11: 583932. 10.3389/fgene.2020.583932. - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

