Outpatient sexually transmitted infection testing and treatment patterns in the United States: a real-world database study
- PMID: 37442964
- PMCID: PMC10339584
- DOI: 10.1186/s12879-023-08434-2
Outpatient sexually transmitted infection testing and treatment patterns in the United States: a real-world database study
Abstract
Background: Chlamydia trachomatis (CT) and Neisseria gonorrhoeae (NG) are the most common notifiable sexually transmitted infections (STIs) in the United States. Because symptoms of these infections often overlap with other urogenital infections, misdiagnosis and incorrect treatment can occur unless appropriate STI diagnostic testing is performed in clinical settings. The objective of this study was to describe STI diagnostic testing and antimicrobial treatment patterns and trends among adolescent and adult men and women with lower genitourinary tract symptoms (LGUTS).
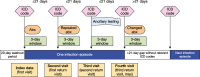
Methods: We analyzed insurance claims data from the IBM® MarketScan® Research Databases. Patients included were between 14 and 64 years old with LGUTS as determined by selected International Classification of Diseases codes between January 2010 and December 2019. Testing of STIs and relevant drug claims were captured, and distribution of testing patterns and drug claims were described.
Results: In total, 23,537,812 episodes with LGUTS (87.4% from women; 12.6% from men) were analyzed from 12,341,154 patients. CT/NG testing occurred in only 17.6% of all episodes. For episodes where patients received treatment within 2 weeks of the visit date, 89.3% received treatment within the first 3 days (likely indicating presumptive treatment), and 77.7% received it on the first day. For women with pelvic inflammatory disease and men with orchitis/epididymitis and acute prostatitis, ≤ 15% received CT/NG testing, and around one-half received antibiotic treatment within 3 days.
Conclusions: Our study revealed low CT/NG testing rates, even in patients diagnosed with complications commonly associated with these STIs, along with high levels of potentially inappropriate presumptive treatment. This highlights the need for timely and accurate STI diagnosis in patients with LGUTS to inform appropriate treatment recommendations.
Keywords: Antimicrobial treatment; Chlamydia trachomatis; Diagnostic testing; Neisseria gonorrhoeae; Sexually transmitted infections.
© 2023. The Author(s).
Conflict of interest statement
ZH, RA, AH, RS and BY are employees of Roche Molecular Systems and report receiving stocks and stock options from Roche. RL reports receiving funding for this study from Roche, receipt of previous grants for clinical trials from Hologic, Visby, OrthoClinical Diagnostics, Becton Dickinson, Cepheid, Merck and Gilead, as well as previous speakers’ bureau payment and support for meeting attendance from Cepheid and reports participation in a Roche advisory board. SNT reports receiving funding for this study from Roche Molecular Systems, as well as receipt of previous grants from Roche Molecular Systems paid directly to their institution. LK reports receiving consulting fees for this study from Roche Diagnostics, as well as receipt of previous consulting fees and medical writing support from Roche Diagnostics.
Figures

References
-
- Centers for Disease Control and Prevention. National overview of STDs, 2021. 2023. https://www.cdc.gov/std/statistics/2021/overview.htm. Accessed 12 May 2023.
-
- Centers for Disease Control and Prevention. Impact of COVID-19 on STDs. 2022. https://www.cdc.gov/std/statistics/2020/impact.htm. Accessed 4 Apr 2023.
-
- U.S. Department of Health and Human Services. Sexually transmitted infections national strategic plan for the United States: 2021–2025. 2020. https://www.hhs.gov/sites/default/files/STI-National-Strategic-Plan-2021.... Accessed 4 Apr 2023.
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

