Management of metastatic melanoma in Texas: disparities in the utilization of immunotherapy following the regulatory approval of immune checkpoint inhibitors
- PMID: 37442992
- PMCID: PMC10339559
- DOI: 10.1186/s12885-023-11142-4
Management of metastatic melanoma in Texas: disparities in the utilization of immunotherapy following the regulatory approval of immune checkpoint inhibitors
Abstract
Background: The utilization of modern-immunotherapies, notably immune checkpoint inhibitors (ICIs), has increased markedly in patients with metastatic melanoma over the past decade and are recommended as standard treatment. Given their increasing adoption in routine care for melanoma, understanding patient access to immunotherapy and patterns of its use in Texas is crucial as it remains one of the few states without Medicaid expansion and with high rates of the uninsured population. The objectives of this study were to examine the trend in the utilization of immunotherapy and to determine factors associated with immunotherapy utilization among patients with metastatic melanoma in the era of ICIs in Texas.
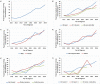
Methods: A retrospective cohort study was conducted using the Texas Cancer Registry (TCR) database. The cohort comprised of adult (≥ 18 years) patients with metastatic melanoma diagnosed between June 2011 and December 2018. The trend in immunotherapy utilization was assessed by determining the proportion of patients receiving immunotherapy each year. The Average Annual Percent Change (AAPC) in immunotherapy utilization was assessed using joinpoint regression, while multivariable logistic regression was used to determine the association between patient characteristics and immunotherapy receipt.
Results: A total of 1,795 adult patients with metastatic melanoma were identified from the TCR. Immunotherapy utilization was higher among younger patients, those with no comorbidities, and patients with private insurance. Multivariable analysis showed that the likelihood of receipt of immunotherapy decreased with older age [(adjusted Odds Ratio (aOR), 0.92; 95% CI, 0.89- 0.93, p = 0.001], living in high poverty neighborhood (aOR, 0.52; 95% CI, 0.44 - 0.66, p < 0.0001), having Medicaid (aOR, 0.58; 95% CI, 0.44 - 0.73, p = 0.02), being uninsured (aOR, 0.49; 95% CI, 0.31 - 0.64, p = 0.01), and having comorbidities (CCI score 1: aOR, 0.48; 95% CI, 0.34 - 0.71, p = 0.003; CCI score ≥ 2: aOR, 0.32; 95% CI, 0.16 - 0.56, p < 0.0001).
Conclusions and relevance: This cohort study identified sociodemographic and socioeconomic disparities in access to immunotherapy in Texas, highlighting the need for policies such as Medicaid expansion that would increase equitable access to this innovative therapy.
Keywords: Access; Disparity; Immune Checkpoint Inhibitors; Immunotherapy; Melanoma; Texas; Utilization.
© 2023. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
Patterns of immunotherapy utilization for non-small cell lung cancer in Texas pre- and post-regulatory approval.Clin Transl Oncol. 2024 Aug;26(8):1908-1920. doi: 10.1007/s12094-024-03412-9. Epub 2024 Mar 30. Clin Transl Oncol. 2024. PMID: 38554190
-
Association of Sociodemographic Factors With Immunotherapy Receipt for Metastatic Melanoma in the US.JAMA Netw Open. 2020 Sep 1;3(9):e2015656. doi: 10.1001/jamanetworkopen.2020.15656. JAMA Netw Open. 2020. PMID: 32876684 Free PMC article.
-
Disparities in Survival Outcomes Among Patients With Metastatic Melanoma in Texas: Implications for Policy and Interventions in the Era of Immune Checkpoint Inhibitors.Am J Clin Oncol. 2024 Nov 1;47(11):517-525. doi: 10.1097/COC.0000000000001128. Epub 2024 Jun 28. Am J Clin Oncol. 2024. PMID: 38937888
-
Mechanisms of resistance to immune checkpoint inhibitors in melanoma: What we have to overcome?Cancer Treat Rev. 2023 Feb;113:102499. doi: 10.1016/j.ctrv.2022.102499. Epub 2022 Dec 13. Cancer Treat Rev. 2023. PMID: 36542945 Review.
-
The efficacy of immune checkpoint inhibitors in elderly patients: a meta-analysis and meta-regression.ESMO Open. 2022 Oct;7(5):100577. doi: 10.1016/j.esmoop.2022.100577. Epub 2022 Sep 23. ESMO Open. 2022. PMID: 36156450 Free PMC article.
Cited by
-
Immune Checkpoint Inhibitors and Survival Disparities by Health Insurance Coverage Among Patients With Metastatic Cancer.JAMA Netw Open. 2025 Jul 1;8(7):e2519274. doi: 10.1001/jamanetworkopen.2025.19274. JAMA Netw Open. 2025. PMID: 40622711 Free PMC article.
-
The Role of Monoclonal Antibodies as Therapeutics in HPV-Related Head and Neck Cancers: An Updated Review.Antibodies (Basel). 2025 Apr 24;14(2):37. doi: 10.3390/antib14020037. Antibodies (Basel). 2025. PMID: 40407689 Free PMC article. Review.
-
Immunotherapy Utilization Outcomes in Distantly Metastatic Head and Neck Squamous Cell Carcinoma.JAMA Otolaryngol Head Neck Surg. 2025 Jul 1;151(7):699-709. doi: 10.1001/jamaoto.2025.1351. JAMA Otolaryngol Head Neck Surg. 2025. PMID: 40471563
References
-
- National Cancer Institute B. SEER Cancer Stat Facts: Melanoma of the Skin . https://seer.cancer.gov/statfacts/html/melan.html. Accessed 19 Jul 2022.
-
- Lim J, Cho E, Lee K, Choi Y, Seo Y, Jeon H, et al. Current immunotherapy approaches for malignant melanoma. BioChip J. 2019;13:105–14. doi: 10.1007/s13206-019-3108-8. - DOI
-
- Vasekar MK, Agbese E, Leslie D. The value of immunotherapy: Comparison of annual cost per patient receiving immunotherapy versus chemotherapy in patients with non-small cell lung cancer. 2020;38 15_suppl: e19364–e19364. 10.1200/JCO20203815_suppl.e19364.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

