Nanosilicate-functionalized nanofibrous membrane facilitated periodontal regeneration potential by harnessing periodontal ligament cell-mediated osteogenesis and immunomodulation
- PMID: 37443072
- PMCID: PMC10339597
- DOI: 10.1186/s12951-023-01982-4
Nanosilicate-functionalized nanofibrous membrane facilitated periodontal regeneration potential by harnessing periodontal ligament cell-mediated osteogenesis and immunomodulation
Abstract
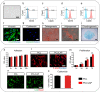
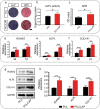
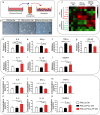
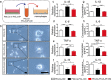
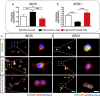
Although various new biomaterials have enriched the methods for periodontal regeneration, their efficacy is still controversial, and the regeneration of damaged support tissue in the periodontium remains challenging. Laponite (LAP) nanosilicate is a layered two-dimensional nanoscale, ultrathin nanomaterial with a unique structure and brilliant biocompatibility and bioactivity. This study aimed to investigate the effects of nanosilicate-incorporated PCL (PCL/LAP) nanofibrous membranes on periodontal ligament cells (PDLCs) in vitro and periodontal regeneration in vivo. A PCL/LAP nanofibrous membrane was fabricated by an electrospinning method. The characterization of PCL/LAP nanofibrous membrane were determined by scanning electron microscopy (SEM), energy dispersive spectrum of X-ray (EDS), inductively coupled plasma mass spectrometry (ICP-MS) and tensile test. The proliferation and osteogenic differentiation of PDLCs on the PCL/LAP nanofibrous membrane were evaluated. A PDLCs and macrophage coculture system was used to explore the immunomodulatory effects of the PCL/LAP nanofibrous membrane. PCL/LAP nanofibrous membrane was implanted into rat calvarial and periodontal defects, and the regenerative potential was evaluated by microcomputed topography (micro-CT) and histological analysis. The PCL/LAP nanofibrous membrane showed good biocompatibility and bioactivity. It enhanced the proliferation and osteogenic differentiation of PDLCs. The PCL/LAP nanofibrous membrane also stimulated anti-inflammatory and pro-remodeling N2 neutrophil formation, regulated inflammatory responses and induced M2 macrophage polarization by orchestrating the immunomodulatory effects of PDLCs. The PCL/LAP nanofibrous membrane promoted rat calvarial defect repair and periodontal regeneration in vivo. LAP nanosilicate-incorporated PCL membrane is capable of mediating osteogenesis and immunomodulation of PDLCs in vitro and accelerating periodontal regeneration in vivo. It could be a promising biomaterial for periodontal regeneration therapy.
Keywords: Immunomodulation; Nanosilicate; Osteogenesis; Periodontal ligament; Periodontal regeneration.
© 2023. The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

