BICC1 drives pancreatic cancer progression by inducing VEGF-independent angiogenesis
- PMID: 37443111
- PMCID: PMC10344882
- DOI: 10.1038/s41392-023-01478-5
BICC1 drives pancreatic cancer progression by inducing VEGF-independent angiogenesis
Erratum in
-
Correction: BICC1 drives pancreatic cancer progression by inducing VEGF-independent angiogenesis.Signal Transduct Target Ther. 2024 Jan 5;9(1):8. doi: 10.1038/s41392-023-01721-z. Signal Transduct Target Ther. 2024. PMID: 38177109 Free PMC article. No abstract available.
Abstract
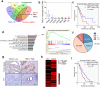
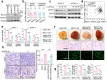
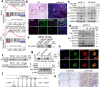
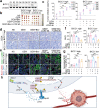
VEGF inhibitors are one of the most successful antiangiogenic drugs in the treatment of many solid tumors. Nevertheless, pancreatic adenocarcinoma (PAAD) cells can reinstate tumor angiogenesis via activation of VEGF-independent pathways, thereby conferring resistance to VEGF inhibitors. Bioinformatic analysis showed that BICC1 was one of the top genes involved in the specific angiogenesis process of PAAD. The analysis of our own cohort confirmed that BICC1 was overexpressed in human PAAD tissues and was correlated to increased microvessel density and tumor growth, and worse prognosis. In cells and mice with xenograft tumors, BICC1 facilitated angiogenesis in pancreatic cancer in a VEGF-independent manner. Mechanistically, as an RNA binding protein, BICC1 bounds to the 3'UTR of Lipocalin-2 (LCN2) mRNA and post-transcriptionally up-regulated LCN2 expression in PAAD cells. When its level is elevated, LCN2 binds to its receptor 24p3R, which directly phosphorylates JAK2 and activates JAK2/STAT3 signal, leading to increased production of an angiogenic factor CXCL1. Blocking of the BICC1/LCN2 signalling reduced the microvessel density and tumor volume of PAAD cell grafts in mice, and increased the tumor suppressive effect of gemcitabine. In conclusion, BICC1 plays a pivotal role in the process of VEGF-independent angiogenesis in pancreatic cancer, leading to resistance to VEGF inhibitors. BICC1/LCN2 signaling may serve as a promising anti-angiogenic therapeutic target for pancreatic cancer patients.
© 2023. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Interleukin 35 Expression Correlates With Microvessel Density in Pancreatic Ductal Adenocarcinoma, Recruits Monocytes, and Promotes Growth and Angiogenesis of Xenograft Tumors in Mice.Gastroenterology. 2018 Feb;154(3):675-688. doi: 10.1053/j.gastro.2017.09.039. Epub 2017 Oct 6. Gastroenterology. 2018. PMID: 28989066
-
Lentivirus-mediated shRNA interference targeting vascular endothelial growth factor inhibits angiogenesis and progression of human pancreatic carcinoma.Oncol Rep. 2013 Mar;29(3):1019-26. doi: 10.3892/or.2012.2203. Epub 2012 Dec 19. Oncol Rep. 2013. PMID: 23254994
-
A study of the suppressive effect on human pancreatic adenocarcinoma cell proliferation and angiogenesis by stable plasmid-based siRNA silencing of c-Src gene expression.Oncol Rep. 2012 Mar;27(3):628-36. doi: 10.3892/or.2011.1602. Epub 2011 Dec 21. Oncol Rep. 2012. PMID: 22200682
-
Targeting Angiogenesis in Cancer Therapy: Moving Beyond Vascular Endothelial Growth Factor.Oncologist. 2015 Jun;20(6):660-73. doi: 10.1634/theoncologist.2014-0465. Epub 2015 May 22. Oncologist. 2015. PMID: 26001391 Free PMC article. Review.
-
Vascular endothelial cell growth factor (VEGF), an emerging target for cancer chemotherapy.Curr Med Chem Anticancer Agents. 2003 Mar;3(2):95-117. doi: 10.2174/1568011033353452. Curr Med Chem Anticancer Agents. 2003. PMID: 12678905 Review.
Cited by
-
Identification of FOXP3+ epithelial cells contributing to pancreatic proliferation and angiogenesis.Am J Physiol Cell Physiol. 2024 Jan 1;326(1):C294-C303. doi: 10.1152/ajpcell.00461.2023. Epub 2023 Dec 4. Am J Physiol Cell Physiol. 2024. PMID: 38047300 Free PMC article.
-
Lymph Node Metastasis in Gastrointestinal Carcinomas: A View from a Proteomics Perspective.Curr Oncol. 2024 Aug 2;31(8):4455-4475. doi: 10.3390/curroncol31080333. Curr Oncol. 2024. PMID: 39195316 Free PMC article. Review.
-
Harnessing Plant Flavonoids to Fight Pancreatic Cancer.Curr Nutr Rep. 2024 Sep;13(3):566-581. doi: 10.1007/s13668-024-00545-9. Epub 2024 May 3. Curr Nutr Rep. 2024. PMID: 38700837 Review.
-
Spatial and single-cell transcriptomics reveal cellular heterogeneity and a novel cancer-promoting Treg cell subset in human clear-cell renal cell carcinoma.J Immunother Cancer. 2025 Jan 4;13(1):e010183. doi: 10.1136/jitc-2024-010183. J Immunother Cancer. 2025. PMID: 39755578 Free PMC article.
-
Microvascular rarefaction caused by the NOTCH signaling pathway is a key cause of TKI-apatinib-induced hypertension and cardiac damage.Front Pharmacol. 2024 Feb 9;15:1346905. doi: 10.3389/fphar.2024.1346905. eCollection 2024. Front Pharmacol. 2024. PMID: 38405666 Free PMC article. Review.
References
-
- Leung DW, et al. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989;246:1306–1309. - PubMed
-
- Carpino G, et al. Thrombospondin 1 and 2 along with PEDF inhibit angiogenesis and promote lymphangiogenesis in intrahepatic cholangiocarcinoma. J. Hepatol. 2021;75:1377–1386. - PubMed
-
- Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. - PubMed
-
- Bergers G, Benjamin LE. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer. 2003;3:401–410. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

