Antipsychotic drug efficacy correlates with the modulation of D1 rather than D2 receptor-expressing striatal projection neurons
- PMID: 37443282
- PMCID: PMC10842629
- DOI: 10.1038/s41593-023-01390-9
Antipsychotic drug efficacy correlates with the modulation of D1 rather than D2 receptor-expressing striatal projection neurons
Abstract
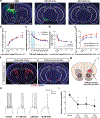
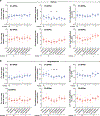
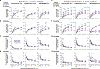
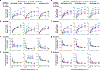
Elevated dopamine transmission in psychosis is assumed to unbalance striatal output through D1- and D2-receptor-expressing spiny-projection neurons (SPNs). Antipsychotic drugs are thought to re-balance this output by blocking D2 receptors (D2Rs). In this study, we found that amphetamine-driven dopamine release unbalanced D1-SPN and D2-SPN Ca2+ activity in mice, but that antipsychotic efficacy was associated with the reversal of abnormal D1-SPN, rather than D2-SPN, dynamics, even for drugs that are D2R selective or lacking any dopamine receptor affinity. By contrast, a clinically ineffective drug normalized D2-SPN dynamics but exacerbated D1-SPN dynamics under hyperdopaminergic conditions. Consistent with antipsychotic effect, selective D1-SPN inhibition attenuated amphetamine-driven changes in locomotion, sensorimotor gating and hallucination-like perception. Notably, antipsychotic efficacy correlated with the selective inhibition of D1-SPNs only under hyperdopaminergic conditions-a dopamine-state-dependence exhibited by D1R partial agonism but not non-antipsychotic D1R antagonists. Our findings provide new insights into antipsychotic drug mechanism and reveal an important role for D1-SPN modulation.
© 2023. The Author(s), under exclusive licence to Springer Nature America, Inc.
Conflict of interest statement
Competing interests
The authors declare no competing interests.
Figures











References
-
- Carlsson A. & Lindqvist M. Effect of chlorpromazine or haloperidol on formation of 3methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol. Toxicol. (Copenh.) 20, 140–144 (1963). - PubMed
-
- Creese I, Burt DR & Snyder SH Dopamine receptor binding predicts clinical and pharmacological potencies of antischizophrenic drugs. Science 192, 481–483 (1976). - PubMed
-
- Matthysse S. Dopamine and the pharmacology of schizophrenia: the state of the evidence. J. Psychiatr. Res. 11, 107–113 (1974). - PubMed
-
- Meltzer HY, Matsubara S. & Lee JC Classification of typical and atypical antipsychotic drugs on the basis of dopamine D-1, D-2 and serotonin2 pKi values. J. Pharmacol. Exp. Ther. 251, 238–246 (1989). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

