Effect of low-dose hydrocortisone and inhaled nitric oxide on inflammatory mediators and local pulmonary metalloproteinases activity in LPS-induced sepsis in piglets
- PMID: 37443327
- PMCID: PMC10344886
- DOI: 10.1038/s41598-023-38311-6
Effect of low-dose hydrocortisone and inhaled nitric oxide on inflammatory mediators and local pulmonary metalloproteinases activity in LPS-induced sepsis in piglets
Abstract
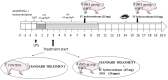
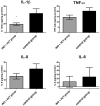
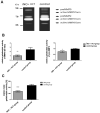
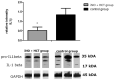
Hospital mortality in sepsis varies between 30-45%. It has been shown that administration of inhaled nitric oxide (iNO) and intravenous corticosteroid in a porcine endotoxemia model attenuated the systemic inflammatory response. We explored the anti-inflammatory effect of a double-treatment strategy (iNO + low-dose steroid) on the lungs in a long-term porcine endotoxic shock model. As metalloproteinases (MMPs) are involved in the initiation of multiple organ dysfunction in septic shock, we evaluated the influence of this combination therapy on MMP2 and MMP9 activity and proIL-1β maturation. A shock-like condition was established in 23 animals by continuous infusion of E. coli lipopolysaccharide (LPS) for 10 h. Then the animals were observed for 10 h. Twelve pigs received iNO and hydrocortisone (iNO treatment started 3 h after the initial LPS infusion and continued until the end of the experiment). Eleven pigs were controls. Pigs treated with iNO and hydrocortisone displayed less inflammatory infiltrates in the lungs than the controls and a lower level of IL-1β. The proMMP2 was significantly decreased in the iNO and hydrocortisone group. The amount of an active MMP9 (~ 60 kDa) was decreased in the iNO and hydrocortisone group. Total gelatinolytic activity was lower in the iNO and hydrocortisone group. Reduced MMP activity was accompanied by a 2.5-fold decrease of the active IL-1β form (17 kDa) in the pulmonary tissue of iNO combined with hydrocortisone exposed pigs. We demonstrated that in a porcine endotoxemia model the NO inhalation combined with intravenous hydrocortisone led to the attenuation of the inflammatory cascade induced by bacterial LPS. The decrease in pulmonary MMPs activities was accompanied by reduced proIL-1β processing.
© 2023. The Author(s).
Conflict of interest statement
Claes Frostell wishes to declare financial interest in the clinical use of inhaled nitric oxide. The other authors declare no conflict of interest.
Figures

Similar articles
-
Organ dysfunction among piglets treated with inhaled nitric oxide and intravenous hydrocortisone during prolonged endotoxin infusion.PLoS One. 2014 May 14;9(5):e96594. doi: 10.1371/journal.pone.0096594. eCollection 2014. PLoS One. 2014. PMID: 24827456 Free PMC article. Clinical Trial.
-
Abnormalities of Coagulation and Fibrinolysis Assessed by Thromboelastometry in an Endotoxic Shock Model in Piglets Treated with Nitric Oxide and Hydrocortisone.Arch Immunol Ther Exp (Warsz). 2024 Jun 7;72(1). doi: 10.2478/aite-2024-0011. eCollection 2024 Jan 1. Arch Immunol Ther Exp (Warsz). 2024. PMID: 38847555
-
The anti-inflammatory effect of inhaled nitric oxide on pulmonary inflammation in a swine model.Can J Physiol Pharmacol. 2005 Mar;83(3):252-8. doi: 10.1139/y05-008. Can J Physiol Pharmacol. 2005. PMID: 15870839
-
Platelet dysfunction in a large-animal model of endotoxic shock; effects of inhaled nitric oxide and low-dose steroid.Nitric Oxide. 2021 Mar 1;108:20-27. doi: 10.1016/j.niox.2020.12.008. Epub 2021 Jan 2. Nitric Oxide. 2021. PMID: 33400993
-
Effect of combined nitric oxide inhalation and NG-nitro-L-arginine infusion in porcine endotoxin shock.Crit Care Med. 1995 May;23(5):909-18. doi: 10.1097/00003246-199505000-00020. Crit Care Med. 1995. PMID: 7736750
Cited by
-
Estimation of normal lung weight index in healthy female domestic pigs.Intensive Care Med Exp. 2024 Jan 26;12(1):6. doi: 10.1186/s40635-023-00591-7. Intensive Care Med Exp. 2024. PMID: 38273120 Free PMC article.
References
-
- Ignarro, L. J., Buga, G. M., Wood, K. S. & Byrns, R. E. Artery and vein is nitric oxide. Proc. Natl. Acad. Sci. [Internet] 84, 9265–9269. http://www.pnas.org/content/84/24/9265.short (1987). - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

