Hyphosphere microorganisms facilitate hyphal spreading and root colonization of plant symbiotic fungus in ammonium-enriched soil
- PMID: 37443341
- PMCID: PMC10504341
- DOI: 10.1038/s41396-023-01476-z
Hyphosphere microorganisms facilitate hyphal spreading and root colonization of plant symbiotic fungus in ammonium-enriched soil
Abstract
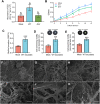
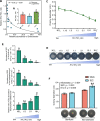
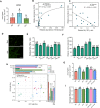
Anthropogenic nitrogen inputs lead to a high ammonium (NH4+)/nitrate (NO3-) ratio in the soil, which restricts hyphal spreading of soil fungi. Access of symbiotic fungi to roots is a prerequisite for plant-fungal interactions. Hyphosphere bacteria protect fungi from environmental stress, yet the impact of hyphosphere bacteria on adaptation of host fungi to NH4+-enriched conditions remains unclear. By developing soil microcosm assays, we report that a plant-symbiotic fungus, Phomopsis liquidambaris, harbors specific hyphosphere bacteria that facilitate hyphal spreading and assist in the root colonization in NH4+-enriched soil. Genetic manipulation, 16S rRNA gene analysis and coinoculation assays revealed that the genus Enterobacter was enriched in the hyphosphere of NH4+-sensitive wild-type compared to NH4+-preferring nitrite reductase-deficient strain. The representative Enterobacter sp. SZ2-promoted hyphal spreading is only evident in nonsterilized soil. We further identified an increased abundance and diversity of ammonia-oxidizing archaea (AOA) and a synchronously decreased NH4+:NO3- ratio following SZ2 inoculation. Microbial supplementation and inhibitor assays showed that AOA-mediated reduction in NH4+:NO3- ratio is responsible for SZ2-enhanced fungal adaptation to NH4+-enriched conditions. The Ph. liquidambaris-Enterobacter-AOA triple interaction promoted rice growth in NH4+-enriched soil. Our study reveals the essential role of hyphosphere microorganism-based hyphal spreading in plant-fungal symbiosis establishment within nitrogen-affected agroecosystems.
© 2023. The Author(s), under exclusive licence to International Society for Microbial Ecology.
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Guether M, Balestrini R, Hannah M, He J, Udvardi MK, Bonfante P. Genome-wide reprogramming of regulatory networks, transport, cell wall and membrane biogenesis during arbuscular mycorrhizal symbiosis in Lotus japonicus. New Phytol. 2009;182:200–12. doi: 10.1111/j.1469-8137.2008.02725.x. - DOI - PubMed
-
- Harinikumar KM, Bagyaraj DJ. Spread of vesicular arbuscular mycorrhizal fungal hyphae in soil. Microbiol Res. 1995;150:77–80. doi: 10.1016/S0944-5013(11)80037-6. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

