Association of NK Cells with the Severity of Fibrosis in Patients with Chronic Hepatitis C
- PMID: 37443584
- PMCID: PMC10340627
- DOI: 10.3390/diagnostics13132187
Association of NK Cells with the Severity of Fibrosis in Patients with Chronic Hepatitis C
Abstract
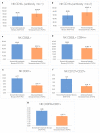
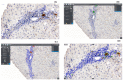
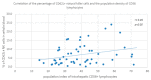
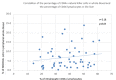
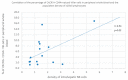
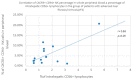
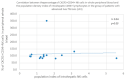
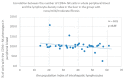
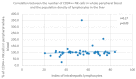
Some NK cell subpopulations may be involved in the modulation of fibrogenesis in the liver. The aim of the study was to evaluate the relationship between the number and phenotype of NK cell subsets in peripheral blood (PB) and total NK cell percentage, population density and the degree of liver fibrosis of patients infected with hepatitis C virus (HCV+). The study group consisted of 56 HCV+ patients, divided into two subgroups: patients with mild or moderate fibrosis and patients with advanced liver fibrosis or cirrhosis (F ≥ 3 in METAVIR classification). The preparations were stained with H-E and AZAN staining. NK cells were targeted with anti-CD56 antibody and identified automatically in situ using the DakoVision system. Assessment of different NK cell subsets in PB was performed with the flow cytometry technique. In the PB of HCV+ patients with advanced liver fibrosis, there was a lower proportion of CD62L+; CD62L+/CD94++; CD27+; CD127+/CD27+ and CXCR3+/CD27+ NK subsets, as compared to patients with mild/moderate liver fibrosis. The results also showed no association between total PB NK cell level and total intrahepatic NK cell population density between patients with mild/moderate fibrosis and with advanced liver fibrosis. However, positive correlations between the PB levels of CD94+ and CD62L+ NK cell subsets and the intrahepatic total NK cell percentage and population density in the liver, irrespectively to the extent of fibrosis, were observed. Additionally, positive correlation was found between the PB CXCR3+/CD94+ NK cell percentages and intrahepatic NK cell percentages in patients with advanced hepatic fibrosis. Lower blood availability of specific NK subsets in patients with chronic type C hepatitis might be a cause of progression of liver fibrosis via insufficient control over hepatic stellate cells.
Keywords: NK cells; chronic hepatitis C; fibrosis; flow cytometry; hepatic stellate cells.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


Similar articles
-
The CXCR3(+)CD56Bright phenotype characterizes a distinct NK cell subset with anti-fibrotic potential that shows dys-regulated activity in hepatitis C.PLoS One. 2012;7(7):e38846. doi: 10.1371/journal.pone.0038846. Epub 2012 Jul 5. PLoS One. 2012. PMID: 22792160 Free PMC article.
-
Natural killer p46High expression defines a natural killer cell subset that is potentially involved in control of hepatitis C virus replication and modulation of liver fibrosis.Hepatology. 2012 Oct;56(4):1201-13. doi: 10.1002/hep.25804. Epub 2012 Aug 31. Hepatology. 2012. PMID: 22532190
-
CD4+ T cells and natural killer cells: Biomarkers for hepatic fibrosis in human immunodeficiency virus/hepatitis C virus-coinfected patients.World J Hepatol. 2017 Sep 8;9(25):1073-1080. doi: 10.4254/wjh.v9.i25.1073. World J Hepatol. 2017. PMID: 28951779 Free PMC article.
-
Natural killer cells in hepatitis C: Current progress.World J Gastroenterol. 2016 Jan 28;22(4):1449-60. doi: 10.3748/wjg.v22.i4.1449. World J Gastroenterol. 2016. PMID: 26819513 Free PMC article. Review.
-
Crosstalk between NK cells and hepatic stellate cells in liver fibrosis (Review).Mol Med Rep. 2022 Jun;25(6):208. doi: 10.3892/mmr.2022.12724. Epub 2022 May 4. Mol Med Rep. 2022. PMID: 35506449 Free PMC article. Review.
Cited by
-
Association of Blood NK Cell Phenotype with the Severity of Liver Fibrosis in Patients with Chronic Viral Hepatitis C with Genotype 1 or 3.Diagnostics (Basel). 2024 Feb 21;14(5):472. doi: 10.3390/diagnostics14050472. Diagnostics (Basel). 2024. PMID: 38472945 Free PMC article.
-
Metabolism of hepatic stellate cells in chronic liver diseases: emerging molecular and therapeutic interventions.Theranostics. 2025 Jan 2;15(5):1715-1740. doi: 10.7150/thno.106597. eCollection 2025. Theranostics. 2025. PMID: 39897543 Free PMC article. Review.
References
-
- Raciborski F., Gujski M., Kłak A., Gierczyński J. HCV w Polsce. Raport Instytutu Ochrony Zdrowia; Warszawa, Poland: 2015.
LinkOut - more resources
Full Text Sources
Research Materials

