SARS-CoV-2 Testing of Emergency Department Patients Using cobas® Liat® and eazyplex® Rapid Molecular Assays
- PMID: 37443639
- PMCID: PMC10341290
- DOI: 10.3390/diagnostics13132245
SARS-CoV-2 Testing of Emergency Department Patients Using cobas® Liat® and eazyplex® Rapid Molecular Assays
Abstract
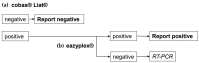
Rapid testing for Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) of patients presenting to emergency departments (EDs) facilitates the decision for isolation on admission to hospital wards. Differences in the sensitivity of molecular assays have implications for diagnostic workflows. This study evaluated the performance of the cobas® Liat® RT-PCR, which is routinely used as the initial test for ED patients in our hospitals, compared with the eazyplex® RT-LAMP. A total of 378 oropharyngeal and nasal swabs with positive Liat® results were analysed. Residual sample aliquots were tested using NeuMoDx™, cobas® RT-PCR, and the eazyplex® assay. Patients were divided into asymptomatic (n = 157) and symptomatic (n = 221) groups according to the WHO case definition. Overall, 14% of positive Liat® results were not confirmed by RT-PCR. These samples were mainly attributed to 26.8% of asymptomatic patients, compared to 3.8% of the symptomatic group. Therefore, positive Liat® results were used to provisionally isolate patients in the ED until RT-PCR results were available. The eazyplex® assay identified 62% and 90.6% of RT-PCR-confirmed cases in asymptomatic and symptomatic patients, respectively. False-negative eazyplex® results were associated with RT-PCR Ct values > 30, and were more frequent in the asymptomatic group than in the symptomatic group (38.1% vs. 5.1%, respectively). Both the Liat® and eazyplex® assays are suitable for testing symptomatic patients. Their use in screening asymptomatic patients depends on the need to exclude any infection or identify those at high risk of transmission.
Keywords: POC; SARS-CoV-2; emergency department; rapid diagnostic test.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Performance of the RT-LAMP-based eazyplex® SARS-CoV-2 as a novel rapid diagnostic test.J Clin Virol. 2021 May;138:104817. doi: 10.1016/j.jcv.2021.104817. Epub 2021 Apr 1. J Clin Virol. 2021. PMID: 33836452 Free PMC article.
-
Clinical Performance of the Point-of-Care cobas Liat for Detection of SARS-CoV-2 in 20 Minutes: a Multicenter Study.J Clin Microbiol. 2021 Jan 21;59(2):e02811-20. doi: 10.1128/JCM.02811-20. Print 2021 Jan 21. J Clin Microbiol. 2021. PMID: 33239382 Free PMC article.
-
Rapid Cobas Liat SARS-CoV-2 Assay in Comparison with the Laboratory-Developed Real-Time RT-PCR Test.Clin Lab. 2021 Nov 1;67(11). doi: 10.7754/Clin.Lab.2021.210316. Clin Lab. 2021. PMID: 34758239
-
Performance comparison of the Cobas® Liat® and Cepheid® GeneXpert® systems on SARS-CoV-2 detection in nasopharyngeal swab and posterior oropharyngeal saliva.Expert Rev Mol Diagn. 2021 May;21(5):515-518. doi: 10.1080/14737159.2021.1919513. Epub 2021 Apr 28. Expert Rev Mol Diagn. 2021. PMID: 33906571 Free PMC article.
-
Point-of-Care Testing for Influenza.2016 Nov 3. In: CADTH Issues in Emerging Health Technologies. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2016–2021. 149. 2016 Nov 3. In: CADTH Issues in Emerging Health Technologies. Ottawa (ON): Canadian Agency for Drugs and Technologies in Health; 2016–2021. 149. PMID: 27977096 Free Books & Documents. Review.
Cited by
-
Phlebotomists on Emergency Department Performance: A Retrospective Comparative Study.Emerg Med Australas. 2025 Jun;37(3):e70081. doi: 10.1111/1742-6723.70081. Emerg Med Australas. 2025. PMID: 40464127 Free PMC article.
-
Detection of SARS-CoV-2 in Settings Including Point-of-Care: Performance of the Cobas SARS-CoV-2 NAT and Utility with the Cobas Liat System.Infect Dis Ther. 2025 Aug;14(8):1815-1827. doi: 10.1007/s40121-025-01186-3. Epub 2025 Jul 14. Infect Dis Ther. 2025. PMID: 40658342 Free PMC article.
References
-
- Kepka S., Ohana M., Séverac F., Muller J., Bayle E., Ruch Y., Laugel E., Oberlin M., Soli M., Hansmann Y., et al. Rapid antigen test combined with chest computed tomography to rule out COVID-19 in patients admitted to the emergency department. J. Clin. Med. 2021;10:3455. doi: 10.3390/jcm10163455. - DOI - PMC - PubMed
-
- Möckel M., Corman V.M., Stegemann M.S., Hofmann J., Stein A., Jones T.C., Gastmeier P., Seybold J., Offermann R., Bachmann U., et al. SARS-CoV-2 antigen rapid immunoassay for diagnosis of COVID-19 in the emergency department. Biomarkers. 2021;26:213–220. doi: 10.1080/1354750X.2021.1876769. - DOI - PMC - PubMed
-
- Bruno A., de Mora D., Freire-Paspuel B., Rodriguez A.S., Paredes-Espinosa M.B., Olmedo M., Sanchez M., Romero J., Paez M., Gonzalez M., et al. Analytical and clinical evaluation of a heat shock SARS-CoV-2 detection method without RNA extraction for N and E genes RT-qPCR. Int. J. Infect. Dis. 2021;109:315–320. doi: 10.1016/j.ijid.2021.06.038. - DOI - PMC - PubMed
-
- Hansen G., Marino J., Wang Z.X., Beavis K.G., Rodrigo J., Labog K., Westblade L.F., Jin R., Love N., Ding K., et al. Clinical performance of the point-of-care cobas Liat for detection of SARS-CoV-2 in 20 minutes: A multicenter study. J. Clin. Microbiol. 2021;59:e02811-20. doi: 10.1128/JCM.02811-20. - DOI - PMC - PubMed
-
- NguyenVan J.C., Gerlier C., Pilmis B., Mizrahi A., Péan de Ponfilly G., Khaterchi A., Enouf V., Ganansia O., Le Monnier A. Prospective evaluation of ID NOW COVID-19 assay used as point-of-care test in an emergency department. J. Clin. Virol. 2021;145:105021. doi: 10.1016/j.jcv.2021.105021. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous