Calcitonin Related Polypeptide Alpha Mediates Oral Cancer Pain
- PMID: 37443709
- PMCID: PMC10341289
- DOI: 10.3390/cells12131675
Calcitonin Related Polypeptide Alpha Mediates Oral Cancer Pain
Abstract
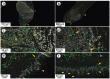
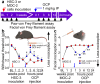
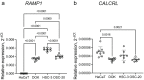
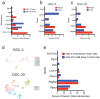
Oral cancer patients suffer pain at the site of the cancer. Calcitonin gene related polypeptide (CGRP), a neuropeptide expressed by a subset of primary afferent neurons, promotes oral cancer growth. CGRP also mediates trigeminal pain (migraine) and neurogenic inflammation. The contribution of CGRP to oral cancer pain is investigated in the present study. The findings demonstrate that CGRP-immunoreactive (-ir) neurons and neurites innervate orthotopic oral cancer xenograft tumors in mice. Cancer increases anterograde transport of CGRP in axons innervating the tumor, supporting neurogenic secretion as the source of CGRP in the oral cancer microenvironment. CGRP antagonism reverses oral cancer nociception in preclinical oral cancer pain models. Single-cell RNA-sequencing is used to identify cell types in the cancer microenvironment expressing the CGRP receptor components, receptor activity modifying protein 1 Ramp1 and calcitonin receptor like receptor (CLR, encoded by Calcrl). Ramp1 and Calcrl transcripts are detected in cells expressing marker genes for Schwann cells, endothelial cells, fibroblasts and immune cells. Ramp1 and Calcrl transcripts are more frequently detected in cells expressing fibroblast and immune cell markers. This work identifies CGRP as mediator of oral cancer pain and suggests the antagonism of CGRP to alleviate oral cancer pain.
Keywords: CALCRL/RAMP1; CGRP; cancer innervation; oral cancer; pain; peptidergic neurons.
Conflict of interest statement
N.W.B. is a founding scientist of Endosome Therapeutics Inc. Research in NWB’s laboratory is partly supported by Takeda Pharmaceuticals International. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

