Multilineage Differentiating Stress Enduring (Muse) Cells: A New Era of Stem Cell-Based Therapy
- PMID: 37443710
- PMCID: PMC10340735
- DOI: 10.3390/cells12131676
Multilineage Differentiating Stress Enduring (Muse) Cells: A New Era of Stem Cell-Based Therapy
Abstract
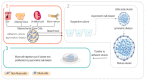
Stem cell transplantation has recently demonstrated a significant therapeutic efficacy in various diseases. Multilineage-differentiating stress-enduring (Muse) cells are stress-tolerant endogenous pluripotent stem cells that were first reported in 2010. Muse cells can be found in the peripheral blood, bone marrow and connective tissue of nearly all body organs. Under basal conditions, they constantly move from the bone marrow to peripheral blood to supply various body organs. However, this rate greatly changes even within the same individual based on physical status and the presence of injury or illness. Muse cells can differentiate into all three-germ-layers, producing tissue-compatible cells with few errors, minimal immune rejection and without forming teratomas. They can also endure hostile environments, supporting their survival in damaged/injured tissues. Additionally, Muse cells express receptors for sphingosine-1-phosphate (S1P), which is a protein produced by damaged/injured tissues. Through the S1P-S1PR2 axis, circulating Muse cells can preferentially migrate to damaged sites following transplantation. In addition, Muse cells possess a unique immune privilege system, facilitating their use without the need for long-term immunosuppressant treatment or human leucocyte antigen matching. Moreover, they exhibit anti-inflammatory, anti-apoptotic and tissue-protective effects. These characteristics circumvent all challenges experienced with mesenchymal stem cells and induced pluripotent stem cells and encourage the wide application of Muse cells in clinical practice. Indeed, Muse cells have the potential to break through the limitations of current cell-based therapies, and many clinical trials have been conducted, applying intravenously administered Muse cells in stroke, myocardial infarction, neurological disorders and acute respiratory distress syndrome (ARDS) related to novel coronavirus (SARS-CoV-2) infection. Herein, we aim to highlight the unique biological properties of Muse cells and to elucidate the advantageous difference between Muse cells and other types of stem cells. Finally, we shed light on their current therapeutic applications and the major obstacles to their clinical implementation from laboratory to clinic.
Keywords: applications; clinical trials; muse cells; unique characteristics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Dezawa M. Muse Cells: Endogenous Reparative Pluripotent Stem Cells. Springer; Berlin/Heidelberg, Germany: New York, NY, USA: 2018.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

