Interactions between the Astrocytic Volume-Regulated Anion Channel and Aquaporin 4 in Hyposmotic Regulation of Vasopressin Neuronal Activity in the Supraoptic Nucleus
- PMID: 37443757
- PMCID: PMC10341125
- DOI: 10.3390/cells12131723
Interactions between the Astrocytic Volume-Regulated Anion Channel and Aquaporin 4 in Hyposmotic Regulation of Vasopressin Neuronal Activity in the Supraoptic Nucleus
Abstract
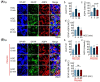
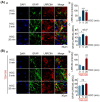
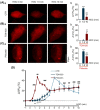
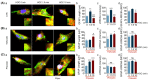
We assessed interactions between the astrocytic volume-regulated anion channel (VRAC) and aquaporin 4 (AQP4) in the supraoptic nucleus (SON). Acute SON slices and cultures of hypothalamic astrocytes prepared from rats received hyposmotic challenge (HOC) with/without VRAC or AQP4 blockers. In acute slices, HOC caused an early decrease with a late rebound in the neuronal firing rate of vasopressin neurons, which required activity of astrocytic AQP4 and VRAC. HOC also caused a persistent decrease in the excitatory postsynaptic current frequency, supported by VRAC and AQP4 activity in early HOC; late HOC required only VRAC activity. These events were associated with the dynamics of glial fibrillary acidic protein (GFAP) filaments, the late retraction of which was mediated by VRAC activity; this activity also mediated an HOC-evoked early increase in AQP4 expression and late subside in GFAP-AQP4 colocalization. AQP4 activity supported an early HOC-evoked increase in VRAC levels and its colocalization with GFAP. In cultured astrocytes, late HOC augmented VRAC currents, the activation of which depended on AQP4 pre-HOC/HOC activity. HOC caused an early increase in VRAC expression followed by a late rebound, requiring AQP4 and VRAC, or only AQP4 activity, respectively. Astrocytic swelling in early HOC depended on AQP4 activity, and so did the early extension of GFAP filaments. VRAC and AQP4 activity supported late regulatory volume decrease, the retraction of GFAP filaments, and subside in GFAP-VRAC colocalization. Taken together, astrocytic morphological plasticity relies on the coordinated activities of VRAC and AQP4, which are mutually regulated in the astrocytic mediation of HOC-evoked modulation of vasopressin neuronal activity.
Keywords: LRRC8A; aquaporin 4; glial fibrillary acidic protein; regulatory volume decrease; volume-regulated anion channels.
Conflict of interest statement
The authors have no conflict of interest to declare.
Figures





References
-
- Escartin C., Galea E., Lakatos A., O’Callaghan J.P., Petzold G.C., Serrano-Pozo A., Steinhauser C., Volterra A., Carmignoto G., Agarwal A., et al. Reactive astrocyte nomenclature, definitions, and future directions. Nat. Neurosci. 2021;24:312–325. doi: 10.1038/s41593-020-00783-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

