Myo/Nog Cells: The Jekylls and Hydes of the Lens
- PMID: 37443759
- PMCID: PMC10340492
- DOI: 10.3390/cells12131725
Myo/Nog Cells: The Jekylls and Hydes of the Lens
Abstract
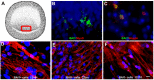
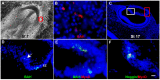
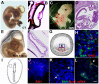
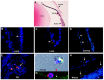
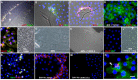
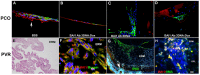
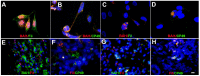
Herein, we review a unique and versatile lineage composed of Myo/Nog cells that may be beneficial or detrimental depending on their environment and nature of the pathological stimuli they are exposed to. While we will focus on the lens, related Myo/Nog cell behaviors and functions in other tissues are integrated into the narrative of our research that spans over three decades, examines multiple species and progresses from early stages of embryonic development to aging adults. Myo/Nog cells were discovered in the embryonic epiblast by their co-expression of the skeletal muscle-specific transcription factor MyoD, the bone morphogenetic protein inhibitor Noggin and brain-specific angiogenesis inhibitor 1. They were tracked from the epiblast into the developing lens, revealing heterogeneity of cell types within this structure. Depletion of Myo/Nog cells in the epiblast results in eye malformations arising from the absence of Noggin. In the adult lens, Myo/Nog cells are the source of myofibroblasts whose contractions produce wrinkles in the capsule. Eliminating this population within the rabbit lens during cataract surgery reduces posterior capsule opacification to below clinically significant levels. Parallels are drawn between the therapeutic potential of targeting Myo/Nog cells to prevent fibrotic disease in the lens and other ocular tissues.
Keywords: BAI1; Myo/Nog; MyoD; Noggin; PCO; PVR; fibrosis; lens; myofibroblasts; retina.
Conflict of interest statement
The authors are co-inventors of a patent describing the use of the G8 mAb to target and isolate BAI1-expressing cells. The authors have no financial relationship surrounding this technology.
Figures
Similar articles
-
Depletion of Myo/Nog Cells in the Lens Mitigates Posterior Capsule Opacification in Rabbits.Invest Ophthalmol Vis Sci. 2019 May 1;60(6):1813-1823. doi: 10.1167/iovs.19-26713. Invest Ophthalmol Vis Sci. 2019. PMID: 31042787
-
Myo/Nog cells are present in the ciliary processes, on the zonule of Zinn and posterior capsule of the lens following cataract surgery.Exp Eye Res. 2018 Jun;171:101-105. doi: 10.1016/j.exer.2018.03.016. Epub 2018 Mar 17. Exp Eye Res. 2018. PMID: 29559302 Free PMC article.
-
Myo/Nog cells: targets for preventing the accumulation of skeletal muscle-like cells in the human lens.PLoS One. 2014 Apr 15;9(4):e95262. doi: 10.1371/journal.pone.0095262. eCollection 2014. PLoS One. 2014. PMID: 24736495 Free PMC article.
-
Myo/Nog cells expressing muscle proteins are present in preretinal membranes from patients with proliferative vitreoretinopathy.Exp Eye Res. 2020 Aug;197:108080. doi: 10.1016/j.exer.2020.108080. Epub 2020 May 29. Exp Eye Res. 2020. PMID: 32474138 Review.
-
Neuroprotective effect of Myo/Nog cells in the stressed retina.Exp Eye Res. 2016 May;146:22-25. doi: 10.1016/j.exer.2015.11.023. Epub 2015 Dec 10. Exp Eye Res. 2016. PMID: 26688580 Review.
References
-
- Choi J., Schultheiss T., Lu M., Wachtler F., Kuruc N., Franke W.W., Bader D., Fischman D.A., Holtzer H. Founder cells for the cardiac and skeletal myogenic lineages. In precursors are critical components of cell fate analyses. In: Stockdale L.H.K.a.F.E., editor. Cellular and Molecular Biology of Muscle Development. A. R. Liss; New York, NY, USA: 1989. pp. 27–36.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

