Impaired Insulin Signaling Alters Mediators of Hippocampal Synaptic Dynamics/Plasticity: A Possible Mechanism of Hyperglycemia-Induced Cognitive Impairment
- PMID: 37443762
- PMCID: PMC10340300
- DOI: 10.3390/cells12131728
Impaired Insulin Signaling Alters Mediators of Hippocampal Synaptic Dynamics/Plasticity: A Possible Mechanism of Hyperglycemia-Induced Cognitive Impairment
Abstract
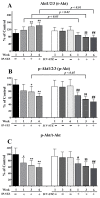
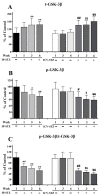
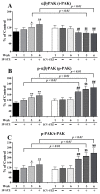
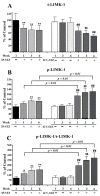
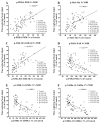
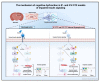
Alzheimer's disease (AD) is a neurological condition that affects the elderly and is characterized by progressive and irreversible neurodegeneration in the cerebral cortex [...].
Keywords: Na+/K+-ATPase; acetylcholine esterase; cognitive behavior; streptozotocin; synapse.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Latina V., Giacovazzo G., Calissano P., Atlante A., La Regina F., Malerba F., Dell’Aquila M., Stigliano E., Balzamino B.O., Micera A., et al. Tau Cleavage Contributes to Cognitive Dysfunction in Strepto-Zotocin-Induced Sporadic Alzheimer’s Disease (sAD) Mouse Model. Int. J. Mol. Sci. 2021;22:2158. doi: 10.3390/ijms222212158. - DOI - PMC - PubMed
-
- Ansari M.A., Rao M.S., Al-Jarallah A., Babiker F.M. Early time course of oxidative stress in hippocampal synaptosomes and cognitive loss following impaired insulin signaling in rats: Development of sporadic Alzheimer’s disease. Brain Res. 2023;1798:148134. doi: 10.1016/j.brainres.2022.148134. - DOI - PubMed
-
- Gao M., Kang Y., Zhang L., Li H., Qu C., Luan X., Liu L., Zhang S. Troxerutin attenuates cognitive decline in the hippocampus of male diabetic rats by inhibiting NADPH oxidase and activating the Nrf2/ARE signaling pathway. Int. J. Mol. Med. 2020;46:1239–1248. doi: 10.3892/ijmm.2020.4653. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

