Cardiovascular Functions of Ena/VASP Proteins: Past, Present and Beyond
- PMID: 37443774
- PMCID: PMC10340426
- DOI: 10.3390/cells12131740
Cardiovascular Functions of Ena/VASP Proteins: Past, Present and Beyond
Abstract
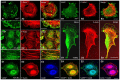
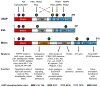
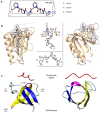
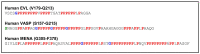
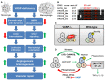
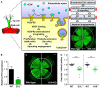
Actin binding proteins are of crucial importance for the spatiotemporal regulation of actin cytoskeletal dynamics, thereby mediating a tremendous range of cellular processes. Since their initial discovery more than 30 years ago, the enabled/vasodilator-stimulated phosphoprotein (Ena/VASP) family has evolved as one of the most fascinating and versatile family of actin regulating proteins. The proteins directly enhance actin filament assembly, but they also organize higher order actin networks and link kinase signaling pathways to actin filament assembly. Thereby, Ena/VASP proteins regulate dynamic cellular processes ranging from membrane protrusions and trafficking, and cell-cell and cell-matrix adhesions, to the generation of mechanical tension and contractile force. Important insights have been gained into the physiological functions of Ena/VASP proteins in platelets, leukocytes, endothelial cells, smooth muscle cells and cardiomyocytes. In this review, we summarize the unique and redundant functions of Ena/VASP proteins in cardiovascular cells and discuss the underlying molecular mechanisms.
Keywords: Ena/VASP proteins; actin dynamics; angiogenesis; cardiomyocyte contraction; conducted vasodilation; endothelial barrier function; gap junction assembly; leukocyte infiltration and polarization; receptor trafficking; smooth muscle cell relaxation.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

