Inflammasomes: Mechanisms of Action and Involvement in Human Diseases
- PMID: 37443800
- PMCID: PMC10340308
- DOI: 10.3390/cells12131766
Inflammasomes: Mechanisms of Action and Involvement in Human Diseases
Abstract
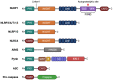
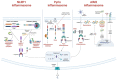
Inflammasome complexes and their integral receptor proteins have essential roles in regulating the innate immune response and inflammation at the post-translational level. Yet despite their protective role, aberrant activation of inflammasome proteins and gain of function mutations in inflammasome component genes seem to contribute to the development and progression of human autoimmune and autoinflammatory diseases. In the past decade, our understanding of inflammasome biology and activation mechanisms has greatly progressed. We therefore provide an up-to-date overview of the various inflammasomes and their known mechanisms of action. In addition, we highlight the involvement of various inflammasomes and their pathogenic mechanisms in common autoinflammatory, autoimmune and neurodegenerative diseases, including atherosclerosis, rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, Alzheimer's disease, Parkinson's disease, and multiple sclerosis. We conclude by speculating on the future avenues of research needed to better understand the roles of inflammasomes in health and disease.
Keywords: autoimmune; autoinflammatory; inflammasome; interleukin 1; neuroinflammatory and neurogenerative disorders; pyroptosis.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Inflammasomes in the pathophysiology of autoinflammatory syndromes.J Leukoc Biol. 2020 Mar;107(3):379-391. doi: 10.1002/JLB.3MIR0919-191R. Epub 2019 Oct 14. J Leukoc Biol. 2020. PMID: 31608507 Free PMC article. Review.
-
Novel insights into the role of inflammasomes in autoimmune and metabolic rheumatic diseases.Rheumatol Int. 2018 Aug;38(8):1345-1354. doi: 10.1007/s00296-018-4074-5. Epub 2018 Jun 4. Rheumatol Int. 2018. PMID: 29869008 Review.
-
NLRP3 Inflammasome: Checkpoint Connecting Innate and Adaptive Immunity in Autoimmune Diseases.Front Immunol. 2021 Oct 11;12:732933. doi: 10.3389/fimmu.2021.732933. eCollection 2021. Front Immunol. 2021. PMID: 34707607 Free PMC article. Review.
-
New Potentiality of Bioactive Substances: Regulating the NLRP3 Inflammasome in Autoimmune Diseases.Nutrients. 2023 Oct 28;15(21):4584. doi: 10.3390/nu15214584. Nutrients. 2023. PMID: 37960237 Free PMC article. Review.
-
Inflammasomes and autoimmune and rheumatic diseases: A comprehensive review.J Autoimmun. 2019 Sep;103:102299. doi: 10.1016/j.jaut.2019.06.010. Epub 2019 Jul 17. J Autoimmun. 2019. PMID: 31326231 Review.
Cited by
-
NLRP3 inflammasome activation and altered mitophagy are key pathways in inclusion body myositis.medRxiv [Preprint]. 2024 Jun 16:2024.06.15.24308845. doi: 10.1101/2024.06.15.24308845. medRxiv. 2024. Update in: J Cachexia Sarcopenia Muscle. 2025 Feb;16(1):e13672. doi: 10.1002/jcsm.13672. PMID: 38947067 Free PMC article. Updated. Preprint.
-
The relationship between neurodevelopmental disorders (NDDs) and NLRP3 inflammasome.Inflamm Res. 2025 Jun 3;74(1):90. doi: 10.1007/s00011-025-02052-1. Inflamm Res. 2025. PMID: 40457054 Review.
-
The Role of NLRP3 Inflammasome in Type 2 Diabetes Mellitus and Its Macrovascular Complications.J Clin Med. 2025 Jun 29;14(13):4606. doi: 10.3390/jcm14134606. J Clin Med. 2025. PMID: 40648980 Free PMC article. Review.
-
Sulphonylureas as Adjunct Therapeutic Agents in the Treatment of Autoimmune Conditions: A Narrative Review.Pharmacol Res Perspect. 2025 Aug;13(4):e70155. doi: 10.1002/prp2.70155. Pharmacol Res Perspect. 2025. PMID: 40686254 Free PMC article. Review.
-
Quercetin Ameliorates Acute Lethal Sepsis in Mice by Inhibiting Caspase-11 Noncanonical Inflammasome in Macrophages.Molecules. 2024 Dec 13;29(24):5900. doi: 10.3390/molecules29245900. Molecules. 2024. PMID: 39769989 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

