Astroglial Cell-to-Cell Interaction with Autoreactive Immune Cells in Experimental Autoimmune Encephalomyelitis Involves P2X7 Receptor, β3-Integrin, and Connexin-43
- PMID: 37443820
- PMCID: PMC10340259
- DOI: 10.3390/cells12131786
Astroglial Cell-to-Cell Interaction with Autoreactive Immune Cells in Experimental Autoimmune Encephalomyelitis Involves P2X7 Receptor, β3-Integrin, and Connexin-43
Abstract
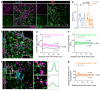
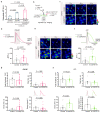
In multiple sclerosis (MS), glial cells astrocytes interact with the autoreactive immune cells that attack the central nervous system (CNS), which causes and sustains neuroinflammation. However, little is known about the direct interaction between these cells when they are in close proximity in the inflamed CNS. By using an experimental autoimmune encephalomyelitis (EAE) model of MS, we previously found that in the proximity of autoreactive CNS-infiltrated immune cells (CNS-IICs), astrocytes respond with a rapid calcium increase that is mediated by the autocrine P2X7 receptor (P2X7R) activation. We now reveal that the mechanisms regulating this direct interaction of astrocytes and CNS-IICs involve the coupling between P2X7R, connexin-43, and β3-integrin. We found that P2X7R and astroglial connexin-43 interact and concentrate in the immediate proximity of the CNS-IICs in EAE. P2X7R also interacts with β3-integrin, and the block of astroglial αvβ3-integrin reduces the P2X7R-dependent calcium response of astrocytes upon encountering CNS-IICs. This interaction was dependent on astroglial mitochondrial activity, which regulated the ATP-driven P2X7R activation and facilitated the termination of the astrocytic calcium response evoked by CNS-IICs. By further defining the interactions between the CNS and the immune system, our findings provide a novel perspective toward expanding integrin-targeting therapeutic approaches for MS treatment by controlling the cell-cell interactions between astrocytes and CNS-IICs.
Keywords: astrocytes; calcium signaling; central nervous system autoimmune disease; hemichannel; immune cell; integrin; multiple sclerosis; purinergic receptors.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Central nervous system-infiltrated immune cells induce calcium increase in astrocytes via astroglial purinergic signaling.J Neurosci Res. 2020 Nov;98(11):2317-2332. doi: 10.1002/jnr.24699. Epub 2020 Aug 16. J Neurosci Res. 2020. PMID: 32799373
-
Early P2X7R-related astrogliosis in autoimmune encephalomyelitis.Mol Cell Neurosci. 2016 Jul;74:1-9. doi: 10.1016/j.mcn.2016.02.003. Epub 2016 Feb 26. Mol Cell Neurosci. 2016. PMID: 26921791
-
Integrin-mediated transactivation of P2X7R via hemichannel-dependent ATP release stimulates astrocyte migration.Biochim Biophys Acta. 2016 Sep;1863(9):2175-88. doi: 10.1016/j.bbamcr.2016.05.018. Epub 2016 May 25. Biochim Biophys Acta. 2016. PMID: 27235833
-
Astroglial and Microglial Purinergic P2X7 Receptor as a Major Contributor to Neuroinflammation during the Course of Multiple Sclerosis.Int J Mol Sci. 2021 Aug 5;22(16):8404. doi: 10.3390/ijms22168404. Int J Mol Sci. 2021. PMID: 34445109 Free PMC article. Review.
-
Astrocytes in multiple sclerosis and experimental autoimmune encephalomyelitis: Star-shaped cells illuminating the darkness of CNS autoimmunity.Brain Behav Immun. 2019 Aug;80:10-24. doi: 10.1016/j.bbi.2019.05.029. Epub 2019 May 21. Brain Behav Immun. 2019. PMID: 31125711 Review.
Cited by
-
Astroglial connexin 43 is a novel therapeutic target for chronic multiple sclerosis model.Sci Rep. 2024 May 13;14(1):10877. doi: 10.1038/s41598-024-61508-2. Sci Rep. 2024. PMID: 38740862 Free PMC article.
-
Mechanistic insights into connexin-mediated neuroglia crosstalk in neurodegenerative diseases.Front Cell Neurosci. 2025 Feb 11;19:1532960. doi: 10.3389/fncel.2025.1532960. eCollection 2025. Front Cell Neurosci. 2025. PMID: 40007760 Free PMC article. Review.
-
Persistent Activation of the P2X7 Receptor Underlies Chronic Inflammation and Carcinogenic Changes in the Intestine.Int J Mol Sci. 2024 Oct 10;25(20):10874. doi: 10.3390/ijms252010874. Int J Mol Sci. 2024. PMID: 39456655 Free PMC article. Review.
References
-
- Walton C., King R., Rechtman L., Kaye W., Leray E., Marrie R.A., Robertson N., La Rocca N., Uitdehaag B., van der Mei I., et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020;26:1816–1821. doi: 10.1177/1352458520970841. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- 451-03-47/2023-01/200007/Ministry of Science, Technological Development and Innovation of Republic of Serbia
- 451-03-47/2023-01/200178/Ministry of Science, Technological Development and Innovation of Republic of Serbia
- 451-03-47/2023-01/200053/Ministry of Science, Technological Development and Innovation of Republic of Serbia
- 101079355/Horizon - EU grant
LinkOut - more resources
Full Text Sources
Medical

