A YAP/TAZ-ARHGAP29-RhoA Signaling Axis Regulates Podocyte Protrusions and Integrin Adhesions
- PMID: 37443829
- PMCID: PMC10340513
- DOI: 10.3390/cells12131795
A YAP/TAZ-ARHGAP29-RhoA Signaling Axis Regulates Podocyte Protrusions and Integrin Adhesions
Abstract
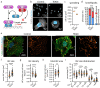
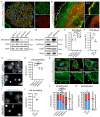
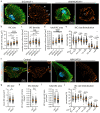
Glomerular disease due to podocyte malfunction is a major factor in the pathogenesis of chronic kidney disease. Identification of podocyte-specific signaling pathways is therefore a prerequisite to characterizing relevant disease pathways and developing novel treatment approaches. Here, we employed loss of function studies for EPB41L5 (Yurt) as a central podocyte gene to generate a cell type-specific disease model. Loss of Yurt in fly nephrocytes caused protein uptake and slit diaphragm defects. Transcriptomic and proteomic analysis of human EPB41L5 knockout podocytes demonstrated impaired mechanotransduction via the YAP/TAZ signaling pathway. Further analysis of specific inhibition of the YAP/TAZ-TEAD transcription factor complex by TEADi led to the identification of ARGHAP29 as an EPB41L5 and YAP/TAZ-dependently expressed podocyte RhoGAP. Knockdown of ARHGAP29 caused increased RhoA activation, defective lamellipodia formation, and increased maturation of integrin adhesion complexes, explaining similar phenotypes caused by loss of EPB41L5 and TEADi expression in podocytes. Detection of increased levels of ARHGAP29 in early disease stages of human glomerular disease implies a novel negative feedback loop for mechanotransductive RhoA-YAP/TAZ signaling in podocyte physiology and disease.
Keywords: ARHGAP29; EPB41L5; YAP/TAZ; Yurt; glomerular kidney disease; mechanotransduction; podocyte.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

