Extraction Efficiency and Alpha-Glucosidase Inhibitory Activities of Green Tea Catechins by Different Infusion Methods
- PMID: 37444349
- PMCID: PMC10340592
- DOI: 10.3390/foods12132611
Extraction Efficiency and Alpha-Glucosidase Inhibitory Activities of Green Tea Catechins by Different Infusion Methods
Abstract
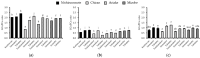
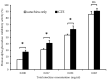
Alpha-glucosidase is an important target for glycemic control with the aim of reducing the risk of type 2 diabetes (T2D). Green tea catechins have been reported to inhibit alpha-glucosidase activity as a potential beverage to control blood glucose levels. However, the effects of the daily infusion style of green tea on tea catechins and their activity remain unclear. In this study, the extraction efficiency of catechins was investigated for 12 green tea extracts (GTEs) infused with 70% ethanol (70% EtOH for 24 h, a favored solvent for catechin extraction), room temperature water infusion (RT H2O for 24 h, an easy way to drink tea), and hot water infusion (Hot H2O for 90 s, a standard way to drink tea). Eight catechins were quantified by HPLC, and the inhibitory effect of GTEs and their catechins on alpha-glucosidase was measured with both rat intestinal enzymes and human Caco-2 cells. The inhibitory mechanism was further analyzed in silico by docking catechins to human alpha-glucosidase using Molecular Operating Environment software. The results showed that total catechins and gallate catechins were efficiently extracted in the order of 70% EtOH, RT H2O, and Hot H2O, and the inhibitory activity against alpha-glucosidase also followed a similar order. Pearson correlation analysis indicated that the alpha-glucosidase inhibitory activity of GTEs was significantly positively correlated with the contents of total catechins, especially gallate catechins. Gallate catechins, such as EGCg and ECg, showed lower IC50 values than free catechins for the enzyme in both rats and humans. In silico simulation revealed that gallate catechins were bound to the different sites with free catechins, and the docking energy of gallate catechins was lower than that of free catechins. Taken together, our data indicated that the daily infusion style of green tea significantly impacted the extraction efficiency and alpha-glucosidase inhibitory activities of catechins, which will give us insight into the use of green tea catechins for glycemic control through efficient infusion.
Keywords: alpha-glucosidase; catechins; gallate catechins; green tea; infusion methods.
Conflict of interest statement
The authors declare no conflict of interest.
Figures








References
-
- Setiawan V., Phangestu S., Soetikno A.G., Arianti A., Kohar I. Rapid Screening Analysis of Antioxidant Activities in Green Tea Products Using DPPH and FRAP. Pharm. J. Indones. 2021;7:9–14. doi: 10.21776/ub.pji.2021.007.01.2. - DOI
LinkOut - more resources
Full Text Sources
Research Materials

