The Relationship between Telomere Length and Nucleoplasmic Bridges and Severity of Disease in Prostate Cancer Patients
- PMID: 37444460
- PMCID: PMC10340763
- DOI: 10.3390/cancers15133351
The Relationship between Telomere Length and Nucleoplasmic Bridges and Severity of Disease in Prostate Cancer Patients
Abstract
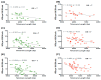
Telomeres are repetitive nucleotide (TTAGGG) sequences that stabilize the chromosome ends and play an important role in the prevention of cancer initiation and progression. Nucleoplasmic bridges (NPBs) are formed when chromatids remain joined together during mitotic anaphase either due to mis-repair of DNA breaks or due to chromatid end fusion as a result of telomere loss or telomere dysfunction. We tested the hypotheses that (i) telomere length (TL) is shorter in prostate cancer (PC) patients relative to healthy age-matched individuals, (ii) TL differs in different stages of PC and (iii) shorter TL is significantly correlated with NPBs formation in PC cases. TL was measured in whole blood by well-established quantitative PCR method and the frequency of NPBs was measured in lymphocytes using cytokinesis-block micronucleus cytome (CBMNcyt) assay. Our results indicate that TL is shorter and NPBs are increased in PC patients relative to age-matched healthy controls. Furthermore, TL was significantly shorter (p = 0.03) in patients with a Gleason score more than 7 and there was also a significant trend of decreasing TL across all three stages (p trend = 0.01; Gleason score <7, 7 and >7). Furthermore, TL was significantly inversely correlated with NPB frequency in PC patients (r = -0.316; p = 0.001) but not in controls (r = 0.163; p = 0.06) and their relationships became stronger with higher Gleason scores. More studies are required that can confirm our observations and explore mechanistic differences in the role of telomeres in NPB formation in PC cases relative to non-cancer cases.
Keywords: Gleason score; nucleoplasmic bridges; prostate cancer (PC); telomere length (TL).
Conflict of interest statement
The authors declare no conflict of interest.
Figures




References
LinkOut - more resources
Full Text Sources

