Examining Final-Administered Medication as a Measure of Data Quality: A Comparative Analysis of Death Data with the Central Cancer Registry in Republic of Korea
- PMID: 37444480
- PMCID: PMC10341326
- DOI: 10.3390/cancers15133371
Examining Final-Administered Medication as a Measure of Data Quality: A Comparative Analysis of Death Data with the Central Cancer Registry in Republic of Korea
Abstract
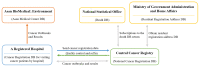
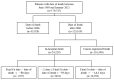
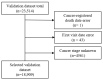
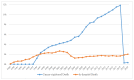
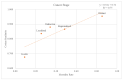
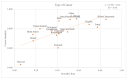
Death is a crucial outcome in retrospective cohort studies, serving as a criterion for analyzing mortality in a database. This study aimed to assess the quality of extracted death data and investigate the potential of the final-administered medication as a variable to quantify accuracy for the validation dataset. Electronic health records from both an in-hospital and the Korean Central Cancer Registry were used for this study. The gold standard was established by examining the differences between the dates of in-hospital deaths and cancer-registered deaths. Cosine similarity was employed to quantify the final-administered medication similarities between the gold standard and other cohorts. The gold standard was determined as patients who died in the hospital after 2006 and whose final hospital visit/discharge date and death date differed by 0 or 1 day. For all three criteria-(a) cancer stage, (b) cancer type, and (c) type of final visit-there was a positive correlation between mortality rates and the similarities of the final-administered medication. This study introduces a measure that can provide additional accurate information regarding death and differentiates the reliability of the dataset.
Keywords: data accuracy; data quality; death; mortality; public data.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gallagher A.M., Dedman D., Padmanabhan S., Leufkens H.G.M., de Vries F. The accuracy of date of death recording in the Clinical Practice Research Datalink GOLD database in England compared with the Office for National Statistics death registrations. Pharmacoepidemiol. Drug Saf. 2019;28:563–569. doi: 10.1002/pds.4747. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

