CTHRC1 Induces Pancreatic Stellate Cells (PSCs) into Myofibroblast-like Cancer-Associated Fibroblasts (myCAFs)
- PMID: 37444482
- PMCID: PMC10340658
- DOI: 10.3390/cancers15133370
CTHRC1 Induces Pancreatic Stellate Cells (PSCs) into Myofibroblast-like Cancer-Associated Fibroblasts (myCAFs)
Abstract
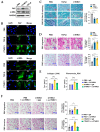
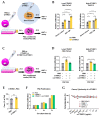
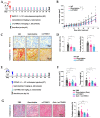
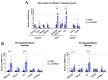
[BACKGROUND] Collagen triple helix repeat containing-1 (CTHRC1) is a secreted protein that contributes to the progression of various cancers, including pancreatic cancer. The higher expression of CTHRC1 in tumor tissues is associated with poorer survival outcomes. However, its specific roles in tumor extracellular matrix (ECM) remodeling remain unclear. Our study aims to investigate the influences of CTHRC1 on pancreatic stellate cells (PSCs), a main source of ECM production in pancreatic cancer. [METHODS AND RESULTS] The analyses of the publicly available pancreatic cancer patient data revealed that CTHRC1 is mainly expressed in cancer stroma and highly correlated with ECM-related genes. An in vitro study showed that more than 40% of these genes can be upregulated by CTHRC1. CTHRC1 specifically activated PSC into myofibroblast-like cancer-associated fibroblasts (myCAFs), which are characterized by a significantly upregulated POSTN gene expression. Periostin (coded by the POSTN gene) has a central role in the CTHRC1-PSCs-cancer metastasis axis. Furthermore, CTHRC1 promoted pancreatic cancer cell proliferation through PSC activation to a greater extent than via direct stimulation. Proof-of-concept experiments showed that the long-term (4-week) inhibition of CTHRC1 led to significant tumor suppression and ECM reduction, and also resulted in an unexpected shift in the CAF subtype from myCAFs to inflammatory CAFs (iCAFs). [CONCLUSION] PSC activation was demonstrated to be the key molecular mechanism responsible for the tumor-promoting effects of CTHRC1, and CTHRC1 has a critical role in CAF subtype differentiation and tumor microenvironment (TME) remodeling. The inhibition of CTHRC1 as a therapeutic strategy for the treatment of pancreatic cancer warrants further investigation.
Keywords: CTHRC1; cancer-associated fibroblast; cell differentiation; extracellular matrix; microenvironment myofibroblast; neoplasm metastasis; pancreatic cancer; pancreatic stellate cell; periostin.
Conflict of interest statement
S.S.K. and M.K.K. are among the patent inventors of the anti-CTHRC1 antibody used in this study. The other authors declare no conflicts of interest. The funder had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the result.
Figures




References
-
- Pyagay P., Heroult M., Wang Q., Lehnert W., Belden J., Liaw L., Friesel R.E., Lindner V. Collagen triple helix repeat containing 1, a novel secreted protein in injured and diseased arteries, inhibits collagen expression and promotes cell migration. Circ. Res. 2005;96:261–268. doi: 10.1161/01.RES.0000154262.07264.12. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

