The Tumor Suppressor DAB2IP Is Regulated by Cell Contact and Contributes to YAP/TAZ Inhibition in Confluent Cells
- PMID: 37444489
- PMCID: PMC10340159
- DOI: 10.3390/cancers15133379
The Tumor Suppressor DAB2IP Is Regulated by Cell Contact and Contributes to YAP/TAZ Inhibition in Confluent Cells
Abstract
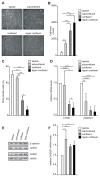
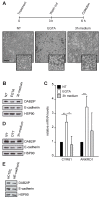
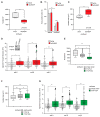
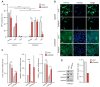
External and internal mechanical forces modulate cell morphology, movement, proliferation and metabolism, and represent crucial inputs for tissue homeostasis. The transcriptional regulators YAP and TAZ are important effectors of mechanical signaling and are frequently activated in solid tumors, correlating with metastasis, chemoresistance, and shorter patient survival. YAP/TAZ activity is controlled by various pathways that sense cell shape, polarity, contacts, and mechanical tension. In tumors, aberrant YAP/TAZ activation may result from cancer-related alterations of such regulatory networks. The tumor suppressor DAB2IP is a Ras-GAP and scaffold protein that negatively modulates multiple oncogenic pathways and is frequently downregulated or inactivated in solid tumors. Here, we provide evidence that DAB2IP expression is sustained by cell confluency. We also find that DAB2IP depletion in confluent cells alters their morphology, reducing cell packing while increasing cell stiffness. Finally, we find that DAB2IP depletion in confluent cells favors YAP/TAZ nuclear localization and transcriptional activity, while its ectopic expression in subconfluent cells increases YAP/TAZ retention in the cytoplasm. Together, these data suggest that DAB2IP may function as a sensor of cell interactions, contributing to dampening cellular responses to oncogenic inputs in confluent cells and that DAB2IP loss-of-function would facilitate YAP/TAZ activation in intact epithelia, accelerating oncogenic transformation.
Keywords: AIP1; Hippo pathway; Ras-GAP; cell stiffness; cell-to-cell contact; contact inhibition; mechanotransduction.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Northey J.J., Przybyla L., Weaver V.M. Tissue Force Programs Cell Fate and Tumor Aggression. Cancer Discov. 2017;7:1224–1237. doi: 10.1158/2159-8290.CD-16-0733. - DOI - PMC - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

