MET Receptor Tyrosine Kinase Inhibition Reduces Interferon-Gamma (IFN-γ)-Stimulated PD-L1 Expression through the STAT3 Pathway in Melanoma Cells
- PMID: 37444518
- PMCID: PMC10340457
- DOI: 10.3390/cancers15133408
MET Receptor Tyrosine Kinase Inhibition Reduces Interferon-Gamma (IFN-γ)-Stimulated PD-L1 Expression through the STAT3 Pathway in Melanoma Cells
Abstract
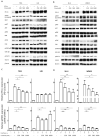
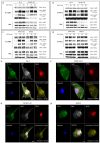
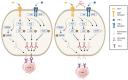
Melanoma is the leading cause of death from cutaneous malignancy. While targeted therapy and immunotherapy with checkpoint inhibitors have significantly decreased the mortality rate of this disease, advanced melanoma remains a therapeutic challenge. Here, we confirmed that interferon-gamma (IFN-γ)-induced PD-L1 expression in melanoma cell lines. This increased expression was down-regulated by the reduction in phosphorylated STAT3 signaling via MET tyrosine kinase inhibitor treatment. Furthermore, immunoprecipitation and confocal immunofluorescence microscopy analysis reveals MET and PD-L1 protein-protein interaction and colocalization on the cell surface membrane of melanoma cells. Together, these findings demonstrate that the IFN-γ-induced PD-L1 expression in melanoma cells is negatively regulated by MET inhibition through the JAK/STAT3 signaling pathway and establish the colocalization and interaction between an RTK and a checkpoint protein in melanoma cells.
Keywords: MET; PD-L1; STAT3; immunotherapy; melanoma; tyrosine kinase inhibitors.
Conflict of interest statement
The authors declare that they have no competing interest.
Figures

Similar articles
-
PD-L1 induced by IFN-γ from tumor-associated macrophages via the JAK/STAT3 and PI3K/AKT signaling pathways promoted progression of lung cancer.Int J Clin Oncol. 2017 Dec;22(6):1026-1033. doi: 10.1007/s10147-017-1161-7. Epub 2017 Jul 26. Int J Clin Oncol. 2017. PMID: 28748356
-
Melanoma response to anti-PD-L1 immunotherapy requires JAK1 signaling, but not JAK2.Oncoimmunology. 2018 Mar 6;7(6):e1438106. doi: 10.1080/2162402X.2018.1438106. eCollection 2018. Oncoimmunology. 2018. PMID: 29872580 Free PMC article.
-
Inducible but not constitutive expression of PD-L1 in human melanoma cells is dependent on activation of NF-κB.PLoS One. 2015 Apr 6;10(4):e0123410. doi: 10.1371/journal.pone.0123410. eCollection 2015. PLoS One. 2015. PMID: 25844720 Free PMC article.
-
The Next Immune-Checkpoint Inhibitors: PD-1/PD-L1 Blockade in Melanoma.Clin Ther. 2015 Apr 1;37(4):764-82. doi: 10.1016/j.clinthera.2015.02.018. Epub 2015 Mar 29. Clin Ther. 2015. PMID: 25823918 Free PMC article. Review.
-
Recent advances of molecular mechanisms of regulating PD-L1 expression in melanoma.Int Immunopharmacol. 2020 Nov;88:106971. doi: 10.1016/j.intimp.2020.106971. Epub 2020 Sep 18. Int Immunopharmacol. 2020. PMID: 33182029 Review.
Cited by
-
Enhancing cancer care with improved checkpoint inhibitors: a focus on PD-1/PD-L1.EXCLI J. 2024 Oct 29;23:1303-1326. doi: 10.17179/excli2024-7783. eCollection 2024. EXCLI J. 2024. PMID: 39624113 Free PMC article. Review.
-
PDL1 targeting by miR-138-5p amplifies anti-tumor immunity and Jurkat cells survival in non-small cell lung cancer.Sci Rep. 2024 Jun 12;14(1):13542. doi: 10.1038/s41598-024-62064-5. Sci Rep. 2024. PMID: 38866824 Free PMC article.
-
Dual role of interferon-gamma in the response of melanoma patients to immunotherapy with immune checkpoint inhibitors.Mol Cancer. 2025 Mar 20;24(1):89. doi: 10.1186/s12943-025-02294-x. Mol Cancer. 2025. PMID: 40108693 Free PMC article. Review.
-
Pan-cancer analysis of MET mutation and its association with the efficacy of immune checkpoint blockade.Genes Dis. 2024 Nov 8;12(4):101450. doi: 10.1016/j.gendis.2024.101450. eCollection 2025 Jul. Genes Dis. 2024. PMID: 40330151 Free PMC article.
-
Editorial for Special Issue "Molecular Mechanisms and Signaling Pathways in Melanoma".Cancers (Basel). 2023 Sep 22;15(19):4675. doi: 10.3390/cancers15194675. Cancers (Basel). 2023. PMID: 37835369 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

