Emerging Biosensing Methods to Monitor Lung Cancer Biomarkers in Biological Samples: A Comprehensive Review
- PMID: 37444523
- PMCID: PMC10341261
- DOI: 10.3390/cancers15133414
Emerging Biosensing Methods to Monitor Lung Cancer Biomarkers in Biological Samples: A Comprehensive Review
Abstract
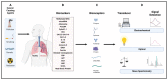
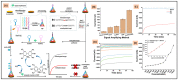
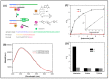
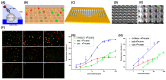
Lung cancer is the most commonly diagnosed of all cancers and one of the leading causes of cancer deaths among men and women worldwide, causing 1.5 million deaths every year. Despite developments in cancer treatment technologies and new pharmaceutical products, high mortality and morbidity remain major challenges for researchers. More than 75% of lung cancer patients are diagnosed in advanced stages, leading to poor prognosis. Lung cancer is a multistep process associated with genetic and epigenetic abnormalities. Rapid, accurate, precise, and reliable detection of lung cancer biomarkers in biological fluids is essential for risk assessment for a given individual and mortality reduction. Traditional diagnostic tools are not sensitive enough to detect and diagnose lung cancer in the early stages. Therefore, the development of novel bioanalytical methods for early-stage screening and diagnosis is extremely important. Recently, biosensors have gained tremendous attention as an alternative to conventional methods because of their robustness, high sensitivity, inexpensiveness, and easy handling and deployment in point-of-care testing. This review provides an overview of the conventional methods currently used for lung cancer screening, classification, diagnosis, and prognosis, providing updates on research and developments in biosensor technology for the detection of lung cancer biomarkers in biological samples. Finally, it comments on recent advances and potential future challenges in the field of biosensors in the context of lung cancer diagnosis and point-of-care applications.
Keywords: DNA methylation; aptasensors; biosensors; lung cancer; lung cancer biomarkers; microRNA; point of care testing; self-health monitoring.
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Kristina S.A., Endarti D., Aditama H. American Cancer Society Global Cancer—Facts & Figures 4th Edition. Am. Cancer Soc. 2018;29:138–144.
-
- Postmus P.E., Kerr K.M., Oudkerk M., Senan S., Waller D.A., Vansteenkiste J., Escriu C., Peters S. Early and Locally Advanced Non-Small-Cell Lung Cancer (NSCLC): ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. 2017;28:iv1–iv21. doi: 10.1093/annonc/mdx222. - DOI - PubMed
-
- Dingemans A.M.C., Früh M., Ardizzoni A., Besse B., Faivre-Finn C., Hendriks L.E., Lantuejoul S., Peters S., Reguart N., Rudin C.M., et al. Small-Cell Lung Cancer: ESMO Clinical Practice Guidelines for Diagnosis, Treatment and Follow-Up. Ann. Oncol. Off. J. Eur. Soc. Med. Oncol. 2021;32:839. doi: 10.1016/j.annonc.2021.03.207. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources

