Efficacy of a HER2-Targeted Thorium-227 Conjugate in a HER2-Positive Breast Cancer Bone Metastasis Model
- PMID: 37444529
- PMCID: PMC10341074
- DOI: 10.3390/cancers15133419
Efficacy of a HER2-Targeted Thorium-227 Conjugate in a HER2-Positive Breast Cancer Bone Metastasis Model
Abstract
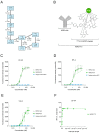
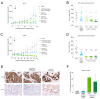
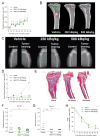
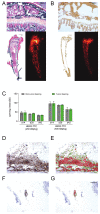
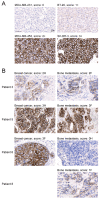
Human epidermal growth factor receptor 2 (HER2) is overexpressed in 15-30% of breast cancers but has low expression in normal tissue, making it attractive for targeted alpha therapy (TAT). HER2-positive breast cancer typically metastasizes to bone, resulting in incurable disease and significant morbidity and mortality. Therefore, new strategies for HER2-targeting therapy are needed. Here, we present the preclinical in vitro and in vivo characterization of the HER2-targeted thorium-227 conjugate (HER2-TTC) TAT in various HER2-positive cancer models. In vitro, HER2-TTC showed potent cytotoxicity in various HER2-expressing cancer cell lines and increased DNA double strand break formation and the induction of cell cycle arrest in BT-474 cells. In vivo, HER2-TTC demonstrated dose-dependent antitumor efficacy in subcutaneous xenograft models. Notably, HER2-TTC also inhibited intratibial tumor growth and tumor-induced abnormal bone formation in an intratibial BT-474 mouse model that mimics breast cancer metastasized to bone. Furthermore, a match in HER2 expression levels between primary breast tumor and matched bone metastases samples from breast cancer patients was observed. These results demonstrate proof-of-concept for TAT in the treatment of patients with HER2-positive breast cancer, including cases where the tumor has metastasized to bone.
Keywords: HER2-positive breast cancer; HER2-targeted therapy; bone metastasis; intratibial mouse model; targeted alpha therapy; targeted radiotherapy; targeted thorium conjugate; thorium-227.
Conflict of interest statement
All authors are current or former employees of Bayer. Christoph Schatz and Alan Cuthbertson are stockholders of Bayer AG. Jenny Karlsson and Alan Cuthbertson hold patents connected to this work. No potential conflicts of interest were disclosed by the other authors.
Figures

References
-
- Hagemann U.B., Wickstroem K., Wang E., Shea A.O., Sponheim K., Karlsson J., Bjerke R.M., Ryan O.B., Cuthbertson A.S. In Vitro and In Vivo Efficacy of a Novel CD33-Targeted Thorium-227 Conjugate for the Treatment of Acute Myeloid Leukemia. Mol. Cancer Ther. 2016;15:2422–2431. doi: 10.1158/1535-7163.MCT-16-0251. - DOI - PubMed
-
- Suominen M.I., Rissanen J.P., Kakonen R., Fagerlund K.M., Alhoniemi E., Mumberg D., Ziegelbauer K., Halleen J.M., Kakonen S.M., Scholz A. Survival benefit with radium-223 dichloride in a mouse model of breast cancer bone metastasis. J. Natl. Cancer Inst. 2013;105:908–916. doi: 10.1093/jnci/djt116. - DOI - PubMed
-
- Supiot S., Faivre-Chauvet A., Couturier O., Heymann M.F., Robillard N., Kraeber-Bodere F., Morandeau L., Mahe M.A., Cherel M. Comparison of the biologic effects of MA5 and B-B4 monoclonal antibody labeled with iodine-131 and bismuth-213 on multiple myeloma. Cancer. 2002;94:1202–1209. doi: 10.1002/cncr.10286. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

