Vitamin D Receptor Antagonist MeTC7 Inhibits PD-L1
- PMID: 37444542
- PMCID: PMC10340436
- DOI: 10.3390/cancers15133432
Vitamin D Receptor Antagonist MeTC7 Inhibits PD-L1
Abstract
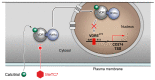
Small-molecule inhibitors of PD-L1 are postulated to control immune evasion in tumors similar to antibodies that target the PD-L1/PD-1 immune checkpoint axis. However, the identity of targetable PD-L1 inducers is required to develop small-molecule PD-L1 inhibitors. In this study, using chromatin immunoprecipitation (ChIP) assay and siRNA, we demonstrate that vitamin D/VDR regulates PD-L1 expression in acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS) cells. We have examined whether a VDR antagonist, MeTC7, can inhibit PD-L1. To ensure that MeTC7 inhibits VDR/PD-L1 without off-target effects, we examined competitive inhibition of VDR by MeTC7, utilizing ligand-dependent dimerization of VDR-RXR, RXR-RXR, and VDR-coactivators in a mammalian 2-hybrid (M2H) assay. MeTC7 inhibits VDR selectively, suppresses PD-L1 expression sparing PD-L2, and inhibits the cell viability, clonogenicity, and xenograft growth of AML cells. MeTC7 blocks AML/mesenchymal stem cells (MSCs) adhesion and increases the efferocytotic efficiency of THP-1 AML cells. Additionally, utilizing a syngeneic colorectal cancer model in which VDR/PD-L1 co-upregulation occurs in vivo under radiation therapy (RT), MeTC7 inhibits PD-L1 and enhances intra-tumoral CD8+T cells expressing lymphoid activation antigen-CD69. Taken together, MeTC7 is a promising small-molecule inhibitor of PD-L1 with clinical potential.
Keywords: AML; M2H assay; MDS; PD-L1; VDR; efferocytosis; small molecule; vitamin D.
Conflict of interest statement
R.K.S. and R.G.M. are listed as the co-inventors on U.S. (11,034,719), EU (3128996), and Canadian (2,982,270) patents assigned to the University of Rochester. The rest of the authors do not carry a conflict of interest.
Figures
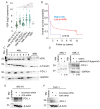
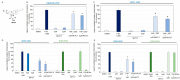


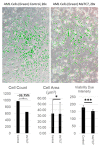
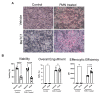

References
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

