Development of the NOGGO GIS v1 Assay, a Comprehensive Hybrid-Capture-Based NGS Assay for Therapeutic Stratification of Homologous Repair Deficiency Driven Tumors and Clinical Validation
- PMID: 37444554
- PMCID: PMC10341077
- DOI: 10.3390/cancers15133445
Development of the NOGGO GIS v1 Assay, a Comprehensive Hybrid-Capture-Based NGS Assay for Therapeutic Stratification of Homologous Repair Deficiency Driven Tumors and Clinical Validation
Abstract
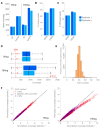
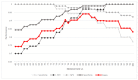
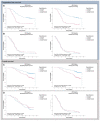
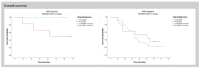
The worldwide approval of the combination maintenance therapy of olaparib and bevacizumab in advanced high-grade serous ovarian cancer requires complex molecular diagnostic assays that are sufficiently robust for the routine detection of driver mutations in homologous recombination repair (HRR) genes and genomic instability (GI), employing formalin-fixed (FFPE) paraffin-embedded tumor samples without matched normal tissue. We therefore established a DNA-based hybrid capture NGS assay and an associated bioinformatic pipeline that fulfils our institution's specific needs. The assay´s target regions cover the full exonic territory of relevant cancer-related genes and HRR genes and more than 20,000 evenly distributed single nucleotide polymorphism (SNP) loci to allow for the detection of genome-wide allele specific copy number alterations (CNA). To determine GI status, we implemented an %CNA score that is robust across a broad range of tumor cell content (25-85%) often found in routine FFPE samples. The assay was established using high-grade serous ovarian cancer samples for which BRCA1 and BRCA2 mutation status as well as Myriad MyChoice homologous repair deficiency (HRD) status was known. The NOGGO (Northeastern German Society for Gynecologic Oncology) GIS (GI-Score) v1 assay was clinically validated on more than 400 samples of the ENGOT PAOLA-1 clinical trial as part of the European Network for Gynaecological Oncological Trial groups (ENGOT) HRD European Initiative. The "NOGGO GIS v1 assay" performed using highly robust hazard ratios for progression-free survival (PFS) and overall survival (OS), as well a significantly lower dropout rate than the Myriad MyChoice clinical trial assay supporting the clinical utility of the assay. We also provide proof of a modular and scalable routine diagnostic method, that can be flexibly adapted and adjusted to meet future clinical needs, emerging biomarkers, and further tumor entities.
Keywords: BRCA1; BRCA2; DNA repair; HRD; HRR; LOH; OS; PAOLA-1; PARP inhibition; PARPi; PFS; breast cancer; diagnostics; genomic instability; homologous repair deficiency; molecular pathology; ovarian cancer; somatic mutation.
Conflict of interest statement
Elena Ioana Braicu MD, PhD receives research funding from: Bayer, Roche Diagnostics, Tesaro, GSK, AstraZeneca and personal fees from: AstraZeneca, Clovis, GSK, Tesaro, EISAI, RochePharma, Roche Diagnostics; Michael Hummel Honorarium: Amgen, AstraZeneca, BMS, Lilly, Novartis, Pfizer, Pierre Fabre, ThermoFisher; Regina Berger receipt of grants/research supports to Institution: Merck, Pharma Mar, AstraZeneca, Kartos Therapeutics, Mersana Therapeutics, Novocure, Clovis Oncology, Seagen Inc.; Vanda Salutari Honorarium: AstraZeneca, MSD Oncology, GSK, PharmaMar and Novocure. Advisory role for Astra Zeneca and Novocure; Markus Tiemann Honorarium/Advisory role for: Astra Zeneca, Boehringer Ingelheim, BMS, MSD, Novartis, Lilly, Roche, Takeda; Lukas C. Heukamp Advisory role for Agilent, Astra Zeneca, Pfizer, BMS, Lilly, Roche.
Figures

References
LinkOut - more resources
Full Text Sources
Miscellaneous

