Identification of a Novel SSTR3 Full Agonist for the Treatment of Nonfunctioning Pituitary Adenomas
- PMID: 37444563
- PMCID: PMC10340464
- DOI: 10.3390/cancers15133453
Identification of a Novel SSTR3 Full Agonist for the Treatment of Nonfunctioning Pituitary Adenomas
Abstract
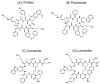
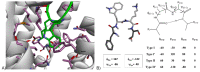
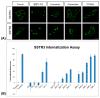
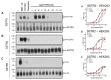
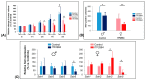
Somatostatin receptor (SSTR) agonists have been extensively used for treating neuroendocrine tumors. Synthetic therapeutic agonists showing selectivity for SSTR2 (Octreotide) or for SSTR2 and SSTR5 (Pasireotide) have been approved for the treatment of patients with acromegaly and Cushing's syndrome, as their pituitary tumors highly express SSTR2 or SSTR2/SSTR5, respectively. Nonfunctioning pituitary adenomas (NFPAs), which express high levels of SSTR3 and show only modest response to currently available SSTR agonists, are often invasive and cannot be completely resected, and therefore easily recur. The aim of the present study was the evaluation of ITF2984, a somatostatin analog and full SSTR3 agonist, as a new potential treatment for NFPAs. ITF2984 shows a 10-fold improved affinity for SSTR3 compared to Octreotide or Pasireotide. Molecular modeling and NMR studies indicated that the higher affinity for SSTR3 correlates with a higher stability of a distorted β-I turn in the cyclic peptide backbone. ITF2984 induces receptor internalization and phosphorylation, and triggers G-protein signaling at pharmacologically relevant concentrations. Furthermore, ITF2984 displays antitumor activity that is dependent on SSTR3 expression levels in the MENX (homozygous mutant) NFPA rat model, which closely recapitulates human disease. Therefore, ITF2984 may represent a novel therapeutic option for patients affected by NFPA.
Keywords: ITF2984; nonfunctioning pituitary adenomas (NFPAs); somatostatin agonists (SSAs); somatostatin receptor 3 (SSTR3).
Conflict of interest statement
S.S. is founder and scientific advisor of 7TM Antibodies GmbH (Jena, Germany); the other authors declare no conflict of interest.
Figures

References
LinkOut - more resources
Full Text Sources

