Emerging Role of Glioma Stem Cells in Mechanisms of Therapy Resistance
- PMID: 37444568
- PMCID: PMC10340782
- DOI: 10.3390/cancers15133458
Emerging Role of Glioma Stem Cells in Mechanisms of Therapy Resistance
Abstract
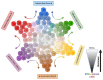
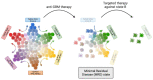
Since their discovery at the beginning of this millennium, glioma stem cells (GSCs) have sparked extensive research and an energetic scientific debate about their contribution to glioblastoma (GBM) initiation, progression, relapse, and resistance. Different molecular subtypes of GBM coexist within the same tumor, and they display differential sensitivity to chemotherapy. GSCs contribute to tumor heterogeneity and recapitulate pathway alterations described for the three GBM subtypes found in patients. GSCs show a high degree of plasticity, allowing for interconversion between different molecular GBM subtypes, with distinct proliferative potential, and different degrees of self-renewal and differentiation. This high degree of plasticity permits adaptation to the environmental changes introduced by chemo- and radiation therapy. Evidence from mouse models indicates that GSCs repopulate brain tumors after therapeutic intervention, and due to GSC plasticity, they reconstitute heterogeneity in recurrent tumors. GSCs are also inherently resilient to standard-of-care therapy, and mechanisms of resistance include enhanced DNA damage repair, MGMT promoter demethylation, autophagy, impaired induction of apoptosis, metabolic adaptation, chemoresistance, and immune evasion. The remarkable oncogenic properties of GSCs have inspired considerable interest in better understanding GSC biology and functions, as they might represent attractive targets to advance the currently limited therapeutic options for GBM patients. This has raised expectations for the development of novel targeted therapeutic approaches, including targeting GSC plasticity, chimeric antigen receptor T (CAR T) cells, and oncolytic viruses. In this review, we focus on the role of GSCs as drivers of GBM and therapy resistance, and we discuss how insights into GSC biology and plasticity might advance GSC-directed curative approaches.
Keywords: glioblastoma; glioma stem cells; heterogeneity; immune checkpoint; plasticity; therapy resistance.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data, in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Stupp R., Hegi M.E., Mason W.P., van den Bent M.J., Taphoorn M.J., Janzer R.C., Ludwin S.K., Allgeier A., Fisher B., Belanger K., et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10:459–466. doi: 10.1016/S1470-2045(09)70025-7. - DOI - PubMed
-
- Stupp R., Taillibert S., Kanner A., Read W., Steinberg D., Lhermitte B., Toms S., Idbaih A., Ahluwalia M.S., Fink K., et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA. 2017;318:2306–2316. doi: 10.1001/jama.2017.18718. - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

