Lactate as Key Metabolite in Prostate Cancer Progression: What Are the Clinical Implications?
- PMID: 37444583
- PMCID: PMC10340474
- DOI: 10.3390/cancers15133473
Lactate as Key Metabolite in Prostate Cancer Progression: What Are the Clinical Implications?
Abstract
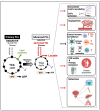
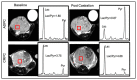
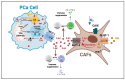
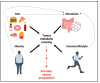
Advanced prostate cancer represents the fifth leading cause of cancer death in men worldwide. Although androgen-receptor signaling is the major driver of the disease, evidence is accumulating that disease progression is supported by substantial metabolic changes. Alterations in de novo lipogenesis and fatty acid catabolism are consistently reported during prostate cancer development and progression in association with androgen-receptor signaling. Therefore, the term "lipogenic phenotype" is frequently used to describe the complex metabolic rewiring that occurs in prostate cancer. However, a new scenario has emerged in which lactate may play a major role. Alterations in oncogenes/tumor suppressors, androgen signaling, hypoxic conditions, and cells in the tumor microenvironment can promote aerobic glycolysis in prostate cancer cells and the release of lactate in the tumor microenvironment, favoring immune evasion and metastasis. As prostate cancer is composed of metabolically heterogenous cells, glycolytic prostate cancer cells or cancer-associated fibroblasts can also secrete lactate and create "symbiotic" interactions with oxidative prostate cancer cells via lactate shuttling to sustain disease progression. Here, we discuss the multifaceted role of lactate in prostate cancer progression, taking into account the influence of the systemic metabolic and gut microbiota. We call special attention to the clinical opportunities of imaging lactate accumulation for patient stratification and targeting lactate metabolism.
Keywords: biomarkers; lactate; metabolic imaging; monocarboxylate transporters; prostate cancer; tumor microenvironment.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Cornford P., Bellmunt J., Bolla M., Briers E., De Santis M., Gross T., Henry A.M., Joniau S., Lam T.B., Mason M.D., et al. EAU-EANM-ESTRO-ESUR-SIOG Guidelines on Prostate Cancer. Part II-2020 Update: Treatment of Relapsing and Metastatic Prostate Cancer. Eur. Urol. 2021;79:263–282. doi: 10.1016/j.eururo.2020.09.046. - DOI - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

