'Stealth' Prostate Tumors
- PMID: 37444597
- PMCID: PMC10341057
- DOI: 10.3390/cancers15133487
'Stealth' Prostate Tumors
Abstract
Background: The aim of this study was to determine the false negative rates of prebiopsy magnetic resonance imaging (MRI) and MRI-ultrasound (US) 12-core systematic prostate biopsy (PBx) by analyzing radical prostatectomy specimens.
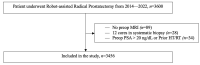
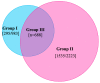
Methods: This retrospective study included 3600 prostate cancer (PCa) patients who underwent robot-assisted laparoscopic radical prostatectomy. Based on comparison of lobe-specific data on final pathology with preoperative biopsy and imaging data, the study population was subdivided into group I-contralateral (CL) benign PBx (n = 983), group II-CL and/or bilateral (BL) non-suspicious mpMRI (n = 2223) and group III-CL benign PBx + non-suspicious mpMRI (n = 688). This population was studied for the presence of PCa, clinically significant PCa (csPCa), extracapsular extension (ECE) (pathological stage pT3), positive frozen section and final positive surgical margin (PSM) in the CL lobe. Descriptive statistics were performed.
Results: In subgroups I, II and III, PCa was respectively detected in 21.5%, 37.7% and 19.5% of cases, and csPCa in 11.3%, 16.3% and 10.3% of cases. CL pT3 disease was seen in 4.5%, 4% and 5.5%, and CL surgical margins and/or frozen section analysis were positive in 6%, 7% and 5% of cases in subgroups I, II and III, respectively.
Conclusions: There are still significant rates of false negatives in the standard care diagnostics of PCa. Further strategies are required to improve the accuracy of diagnosis and determination of tumor location.
Keywords: magnetic resonance imaging; prostate biopsy; prostate cancer.
Conflict of interest statement
Dr. Ash Tewari has served as a site PI on pharma-/industry-sponsored clinical trials from Kite Pharma, Lumicell Inc., Dendreon and Oncovir Inc. His institution has received research funding (grants) from DOD, NIH, Axogen, Intuitive Surgical, AMBFF and other philanthropical organizations. Dr. A.K. Tewari has served as an unpaid consultant for Roivant Biosciences and advisor to Promaxo. He owns equity in Promaxo. The rest of the authors do not have conflict of interest.
Figures


References
LinkOut - more resources
Full Text Sources
Miscellaneous

