The Effectiveness of Compression Garments for Reducing Pain in Non-Vascular Ehlers-Danlos Syndromes: A Prospective Observational Cohort Study
- PMID: 37444695
- PMCID: PMC10340571
- DOI: 10.3390/healthcare11131862
The Effectiveness of Compression Garments for Reducing Pain in Non-Vascular Ehlers-Danlos Syndromes: A Prospective Observational Cohort Study
Abstract
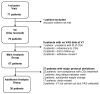
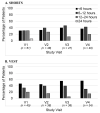
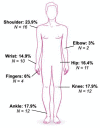
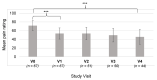
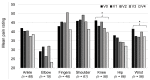
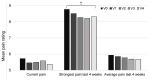
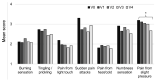
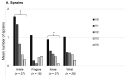
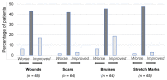
Patients with Ehlers-Danlos Syndrome (EDS) frequently suffer from severe chronic pain. We carried out an observational cohort study to assess the effectiveness of compression garments (CGs) for reducing this pain. Patients with non-vascular EDS were given custom-made Cerecare® CGs during a visit to a specialist clinic (visit V0). They were followed up over 2 years with visits every 6 months (V1-V4). At each visit, pain was assessed for the joints treated with CGs using a visual analogue scale (VAS; 0-100 mm). Additional measures were obtained to assess neuropathic pain (painDETECT questionnaire), proprioception/balance (Berg Balance Scale), and functional independence, amongst others. Data were analyzed for 67 patients with EDS (hypermobile: 91%; classical: 6%; kyphoscoliotic: 3%). For the most painful joint, the mean VAS rating was 71.5 ± 22.8 mm at V0; this decreased to 53.5 ± 25.5 mm at V1 and 45.7 ± 29 mm at V4 (t-tests: p < 0.0001). From V0 to V4, improvements were also seen for pain at the other joints, neuropathic pain, functional independence, proprioception/balance, and the incidence of sprains and dislocations/subluxations, although not all comparisons were statistically significant (p < 0.05 level). These results indicate that CGs may effectively reduce the pain and joint instability in non-vascular EDS patients.
Keywords: Ehlers-Danlos syndromes; compression garments; dislocation; joint hypermobility; joint instability; orthoses; pain therapy; proprioception; quality of life; sprains.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Figures



References
-
- Tinkle B., Castori M., Berglund B., Cohen H., Grahame R., Kazkaz H., Levy H. Hypermobile Ehlers-Danlos Syndrome (a.k.a. Ehlers-Danlos Syndrome Type III and Ehlers-Danlos Syndrome Hypermobility Type): Clinical Description and Natural History. Am. J. Med. Genet. Part C Semin. Med. Genet. 2017;175:48–69. doi: 10.1002/ajmg.c.31538. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

